TrimTACs-Trim21 based molecular gels and PROTAC degradation agents
Targeted protein degradation (TPD) has revolutionized drug discovery by enabling the elimination of disease-causing proteins. However, current strategies relying on E3 ligases like CRBN and VHL lack selectivity for multimeric protein assemblies, which are implicated in diseases such as neurodegeneration, cancer, and autoimmunity. TRIM21, an interferon-inducible E3 ligase, to selectively degrade multimeric proteins in biomolecular condensates. Their work combines serendipitous discovery and rational design to develop TrimTACs, a new class of degraders with unique selectivity for clustered targets.
Key Findings
- Acepromazine's Interferon-Enhanced Cytotoxicity:
- The antipsychotic drug acepromazine (ACE) exhibited potent, interferon (IFNγ/IFNβ)-dependent cytotoxicity in cancer cells expressing aldo-keto reductases (AKR1C1/2/3). These enzymes metabolize ACE into (S)-hydroxy-acepromazine [(S)-ACE-OH], the active stereoisomer.
- IFNγ upregulated TRIM21, an E3 ligase, which mediated ACE's anticancer activity. CRISPR screens confirmed TRIM21 as essential for ACE-induced cell death.
- Mechanism of Nuclear Pore Complex Degradation:
- (S)-ACE-OH recruited TRIM21 to the nuclear pore complex (NPC), triggering ubiquitination and proteasomal degradation of inner-ring proteins (e.g., NUP35, NUP155).
- Structural studies revealed that (S)-ACE-OH binds a hydrophobic pocket in TRIM21's PRYSPRY domain, enabling proximity to NPC subunits. Mutations in NUP98, a key NPC protein, conferred resistance to ACE by disrupting TRIM21 recruitment.
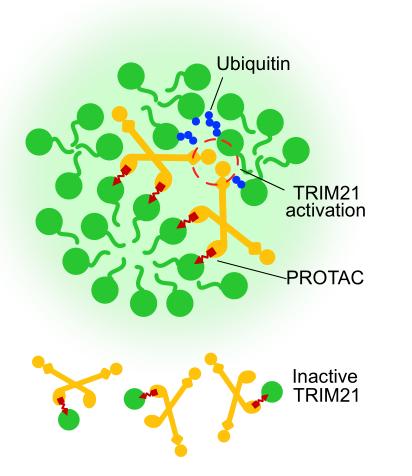
- Design of TRIM21-Based PROTACs (TrimTACs):
- Leveraging structural insights, the authors engineered TrimTACs—heterobifunctional molecules linking ACE derivatives to ligands like JQ1 (targeting BRD4) or SLF (targeting FKBP12).
- TrimTACs selectively degraded multimeric proteins in biomolecular condensates (e.g., PML nuclear bodies, Cajal bodies) while sparing monomeric proteins. This selectivity arises from TRIM21's requirement for substrate-induced clustering to activate its RING domain.
- In Vivo and Pharmacological Validation:
- TrimTACs exhibited favorable pharmacokinetics in mice, with no toxicity observed at therapeutic doses.
- Degradation depended on lysosomal function and retained specificity even in cross-species models.
Therapeutic and Research Implications
- Disease Relevance:
- Aberrant protein aggregation drives pathologies like Alzheimer's disease and amyotrophic lateral sclerosis. TrimTACs offer a strategy to dismantle toxic aggregates without affecting functional monomers.
- In cancer, TrimTACs could disrupt oncogenic condensates (e.g., PML bodies in leukemia) or enhance immunotherapy by degrading immune checkpoints like PD-L1.
- Advantages Over Existing TPD Technologies:
- Tissue Specificity: TRIM21's interferon-inducible expression allows degradation to be restricted to inflamed or cancerous tissues.
- Multimer Selectivity: Unlike CRBN/VHL-based degraders, TrimTACs exploit substrate clustering, minimizing off-target effects on monomeric proteins.
- Structural and Mechanistic Insights:
- Co-crystal structures of TRIM21 with ACE metabolites revealed a ligandable pocket in the PRYSPRY domain, enabling rational PROTAC design.
- The requirement for substrate-induced TRIM21 dimerization provides a built-in mechanism for selectivity, ensuring activity only against multimeric targets.
The modularity of TrimTACs opens avenues for targeting diverse condensate-associated pathologies, from neurodegeneration to cancer. Future work will focus on optimizing pharmacokinetics, expanding target scope, and translating these findings into clinical therapies. The integration of TRIM21 into the TPD toolkit represents a paradigm shift, offering unmatched selectivity for pathogenic protein assemblies. This work underscores the potential of leveraging innate immune pathways for therapeutic innovation.
Reference
- Lu P, Cheng Y, Xue L, Ren X, Xu X, Chen C, Cao L, Li J, Wu Q, Sun S, Hou J, Jia W, Wang W, Ma Y, Jiang Z, Li C, Qi X, Huang N, Han T. Selective degradation of multimeric proteins by TRIM21-based molecular glue and PROTAC degraders. Cell. 2024 Dec 12;187(25):7126-7142.e20. doi: 10.1016/j.cell.2024.10.015. Epub 2024 Nov 1. PMID: 39488207.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

