A novel approach for the fabrication of biocompatible adsorbents
Uremic toxins, particularly protein-bound uremic toxins (PBUTs), are a significant contributor to cardiovascular risk in patients with end-stage chronic kidney disease (CKD). However, conventional hemodialysis fails to efficiently remove these toxins due to their hydrophobic nature and strong binding to plasma proteins.
The researchers chose ferritin, a naturally occurring protein cage composed of 24 identical subunits that self-assemble into a spherical structure with an outer diameter of 12-13 nm and an inner cavity of 6-8 nm, as the building block for their adsorbents. Apoferritin variants, devoid of the iron oxide core, were used to form the materials. A negatively charged outer surface was engineered to prevent the adsorption of negatively charged plasma proteins, such as albumin, which could block the pores and reduce the adsorption efficiency.
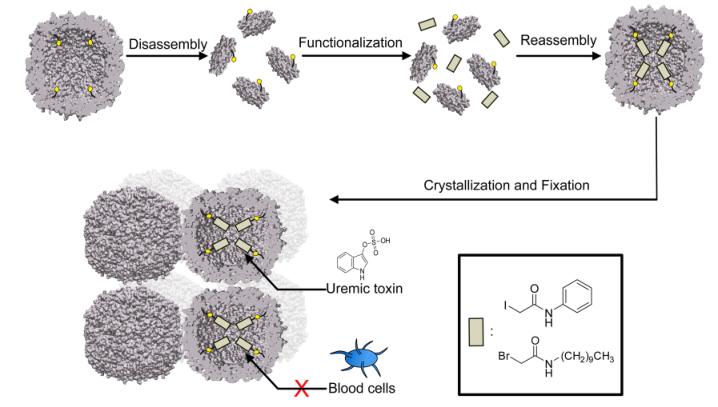
To enhance the toxin binding capacity of the ferritin cages, the inner surface was selectively modified using protein redesign techniques. Cysteine residues were introduced at strategic positions to serve as anchor sites for chemical derivatization. Native cysteine residues were mutated to alanine to prevent unwanted modifications. Two types of hydrophobic ligands, phenylic (Phe) and aliphatic (C10), were conjugated to the cysteine thiol groups, resulting in up to 96 molecules per cage.
The functionalized ferritin cages were assembled into macroscopic crystalline materials using a batch crystallization technique. These materials exhibited uniformly distributed solvent channels and possessed a cubic lattice structure. To stabilize the crystals and enhance their tolerance to aqueous solutions with high protein concentrations, they were cross-linked using sulfo-SMCC, a linker that does not self-react, thus maintaining the adsorption capacity.
Adsorption assays revealed that the unfunctionalized ferritin-based material already showed moderate adsorption capacity for the tested PBUTs, including indoxyl sulfate (IS), p-cresyl sulfate (pCS), and phenylacetic acid (PheAc). Functionalization with phenylic molecules significantly increased the adsorption capacity for IS by about 50%, reaching 458 μg g⁻¹. Although functionalization with aliphatic molecules also improved the adsorption capacity, the effect was less pronounced. No significant enhancement was observed for PheAc adsorption.
Importantly, the biohybrid materials demonstrated high biocompatibility, showing no contamination with endotoxins and no activation of blood platelets upon incubation. These findings suggest that the protein-based adsorbents have great potential for the clearance of uremic toxins, offering a promising alternative to conventional adsorbents that often struggle with biocompatibility issues.
In conclusion, the study demonstrates the successful synthesis of macroscopic materials based on the bottom-up assembly of ferritin protein nanocages for blood purification applications. The introduction of anchor sites for chemical modification allowed the incorporation of hydrophobic molecules within the cage interior, enhancing toxin adsorption without compromising biocompatibility. While further optimization is needed, this work paves the way for the development of novel, highly biocompatible adsorbents for the removal of PBUTs in dialysis patients.
Reference
- Böhler H, Orth-Alampour S, Baaten C, Riedner M, Jankowski J, Beck T. Assembly of chemically modified protein nanocages into 3D materials for the adsorption of uremic toxins. J Mater Chem B. 2022 Dec 22;11(1):55-60. doi: 10.1039/d2tb02386e. PMID: 36504125.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

