IBGs-A Paradigm Shift in Targeted Protein Degradation
Targeted protein degradation (TPD) has revolutionized drug discovery by enabling the elimination of disease-causing proteins. PROTACs and molecular glues dominate this field, but their reliance on trans-binding or single-site interactions limits scope. This study unveils intramolecular bivalent glues (IBGs), a groundbreaking approach that exploits cis-binding to induce degradation, offering unprecedented precision and versatility.
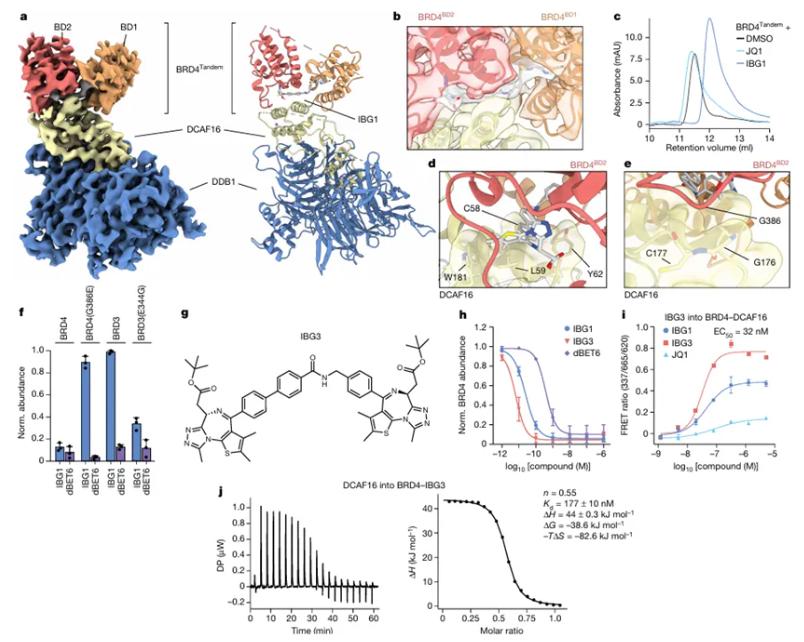
Traditional PROTACs are bifunctional molecules linking a target protein to an E3 ligase. In contrast, IBGs bind two domains within the same protein, inducing structural changes that expose neo-interfaces for E3 ligase recruitment. The study's cryo-EM structure of the BRD4–IBG1–DCAF16 complex illustrates this: IBG1 bridges BRD4's bromodomains, creating a composite surface that docks DCAF16. This mechanism leverages weak intrinsic interactions between BRD4 and DCAF16, amplified by IBG1's bivalent engagement. Such conformational "gluing" bypasses the need for high-affinity E3 ligase binders, a major hurdle for PROTACs.
The researchers employed orthogonal CRISPR screens to identify DCAF16 and DCAF11 as critical E3 ligases. Biophysical assays (e.g., TR-FRET, ITC) confirmed ternary complex formation, while proteomics validated target specificity. Structural insights drove the rational design of IBG3, which replaced E7820 with a second JQ1 moiety, enhancing avidity and achieving sub-nanomolar potency. This exemplifies how structure-guided optimization can refine IBGs for clinical use.
IBGs' ability to recruit distinct E3 ligases (e.g., DCAF11 via IBG4) expands their applicability. Since many proteins are multidomain, IBGs could target transcription factors, kinases, or scaffolding proteins previously deemed undruggable. For example, IBG3's picomolar efficacy against BRD4 suggests promise in cancers driven by BET protein dysregulation, such as leukemia or solid tumors.
While IBGs offer immense potential, challenges remain. Optimizing linkers to balance rigidity and flexibility is critical for domain alignment. Off-target effects may arise from unintended protein-protein interactions, necessitating rigorous selectivity studies. Future work should explore IBGs for non-BET targets and assess in vivo efficacy and toxicity.
IBGs represent a paradigm shift in TPD, transforming how we approach protein degradation. By harnessing intramolecular interactions, they overcome limitations of PROTACs and molecular glues, enabling precise targeting of multidomain proteins. As structural biology and synthetic chemistry advance, IBGs could unlock new therapeutic avenues, redefining the landscape of precision medicine. This study not only elucidates a novel degradation mechanism but also sets the stage for a new generation of degraders with transformative potential in oncology and beyond.
Reference
- Hsia, O., Hinterndorfer, M., Cowan, A.D. et al. Targeted protein degradation via intramolecular bivalent glues. Nature 627, 204–211 (2024). https://doi.org/10.1038/s41586-024-07089-6
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

