NLTC-Bifunctional Nanoplatform for Tumor-Selective Protein Degradation and Enhanced Cancer Immunotherapy
Cancer therapy faces challenges such as tumor heterogeneity, immunosuppressive microenvironments, and limited efficacy of single-modality treatments. Lysosome-targeting chimeras (LYTACs) emerged as a promising strategy to degrade extracellular and membrane proteins, but their clinical translation is hindered by poor tumor accumulation and off-target effects. This study introduces a bifunctional LYTAC nanoplatform (NLTC) that combines tumor-selective protein degradation with immunomodulation to address these limitations.
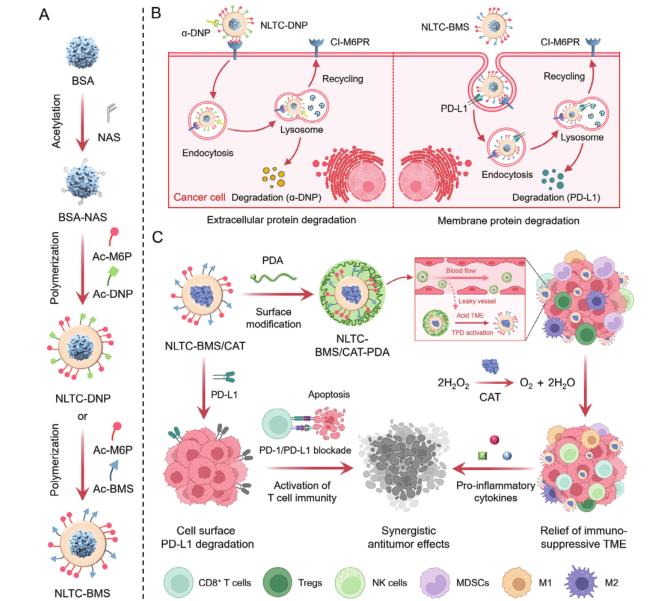
The NLTC is engineered as a protein nanocapsule with a customizable surface. Key components include:
- Targeting Ligands: Acrylated M6P (Ac-M6P) binds to the cation-independent mannose 6-phosphate receptor (CI-M6PR) on cancer cells, facilitating lysosomal delivery.
- Protein Degradation Moieties: Ligands like Ac-BMS target PD-L1, a membrane protein critical for immune evasion.
- Catalase (CAT) Encapsulation: CAT decomposes tumor-associated H₂O₂ into O₂, alleviating hypoxia and inflammation in the tumor microenvironment (TME).
- pH-Responsive PEG Shell: A detachable polyethylene glycol (PEG) coating ensures systemic stability and tumor-specific activation in acidic TME (pH 6.5–6.8).
Key Findings
- Efficient Protein Degradation:
- In vitro, NLTC-BMS degraded over 60% of PD-L1 on B16F10 melanoma cells via CI-M6PR-mediated clathrin-dependent endocytosis. Proteomic analysis confirmed specificity, with minimal off-target effects.
- In vivo, intratumoral NLTC-BMS reduced PD-L1 levels, enhanced CD8⁺ T cell infiltration (31.4% vs. 11.7% in controls), and suppressed tumor growth in B16F10-bearing mice.
- Synergistic Immunomodulation:
- Encapsulated CAT retained 86.6% activity, breaking down H₂O₂ and elevating O₂ levels, which downregulated hypoxia-inducible factor-1a (HIF-1a) and reduced immunosuppressive cells (e.g., Tregs, MDSCs).
- The dual-action NLTC-BMS/CAT-PDA, administered intravenously, achieved 87.9% tumor inhibition and prolonged survival (>50% at 40 days) by synergizing PD-L1 degradation with TME remodeling.
- Tumor-Selective Activation:
- The pH-responsive PEG shell prevented premature activation in normal tissues. NLTC-BMS/CAT-PDA showed 2.35-fold higher tumor accumulation than non-PEGylated versions, with negligible systemic toxicity.
- Long-Term Immune Memory:
- Treated mice exhibited elevated effector and central memory T cells (TEM/TCM) in spleens, reducing lung metastasis and protecting against tumor rechallenge.
This study demonstrates that NLTC integrates targeted protein degradation and immunomodulation, overcoming limitations of traditional LYTACs and monotherapies. However, challenges remain:
- Specificity: Acidic conditions in non-tumor tissues (e.g., inflamed areas) may trigger off-target effects. Future designs could incorporate multi-stimuli responsiveness (e.g., enzymes, ROS).
- Translation: Humanized models are needed to validate efficacy and safety before clinical trials.
The NLTC platform represents a paradigm shift in cancer therapy, merging precision protein degradation with microenvironment modulation. By harnessing nanotechnology and lysosomal targeting, this approach offers a versatile strategy to enhance immunotherapy outcomes, paving the way for next-generation LYTAC-based treatments.
Reference
- Xing Y, Li J, Wang L, Zhu Z, Yan J, Liu Y, Liu Q. A Bifunctional Lysosome-Targeting Chimera Nanoplatform for Tumor-Selective Protein Degradation and Enhanced Cancer Immunotherapy. Adv Mater. 2025 Mar;37(10):e2417942. doi: 10.1002/adma.202417942. Epub 2025 Jan 31. PMID: 39888098.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

