B Cell Markers
Related Symbol Search List
Immunology Background
Background
B cell markers are molecules expressed on the surface of B cells or associated with their function. These markers play a crucial role in the identification, classification, and characterization of B cells. They are often used in research, clinical diagnostics, and immunology studies to distinguish B cells from other cell types and to analyze the different stages of B cell development and activation. Here's an explanation of what B cell markers are and their importance:
| Importance | Details |
|---|---|
| Identification of B Cells |
|
| Characterization of B Cell Subsets |
|
| Stage-Specific Markers |
|
| Functional Markers |
|
B cell markers are vital tools for the identification, characterization, and study of B cells in various contexts. They help researchers and clinicians distinguish B cells from other immune cell types, identify specific B cell subsets, assess B cell development stages, and investigate B cell activation and differentiation. By utilizing these markers, scientists can gain insights into the biology, function, and dysregulation of B cells in health and disease, leading to advancements in immunology and therapeutic approaches targeting B cells.
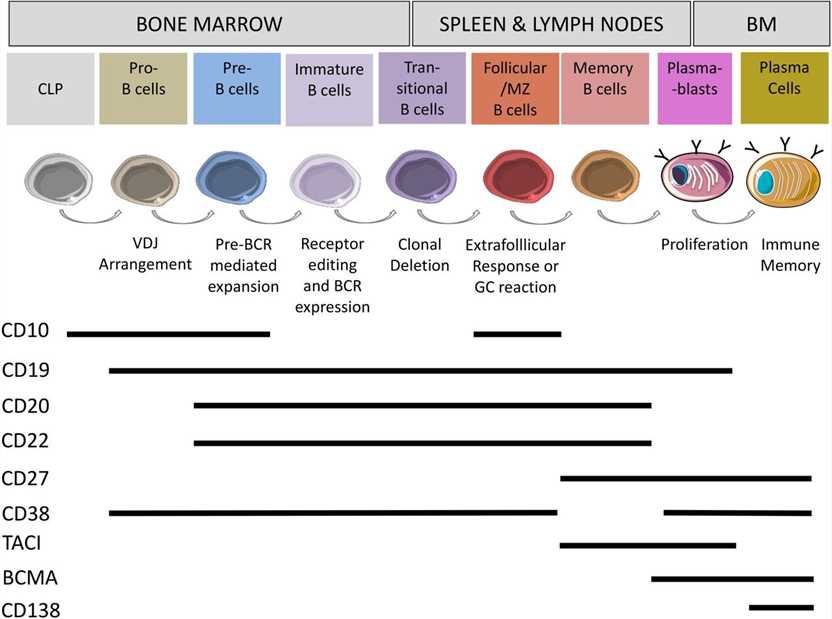 Fig.1 Surface markers on human B cells B cells develop in the bone marrow from haematopoietic stem cells to plasma cells. (Fischer U, et al., 2024)
Fig.1 Surface markers on human B cells B cells develop in the bone marrow from haematopoietic stem cells to plasma cells. (Fischer U, et al., 2024)Key B Cell Markers
These markers represent a range of molecules involved in B cell development, activation, co-stimulation, and antigen recognition. The classification of these markers is as follows:
| Types | Functions |
|---|---|
| B cell receptor-related |
|
| B cell-specific markers |
|
| B cell activation and co-stimulatory molecules |
|
| Co-stimulatory molecules |
|
| Complement-related molecules |
|
| Other B cell markers |
|
Clinical Applications of B Cell Markers
B-Cell Acute Lymphoblastic Leukemia (B-ALL)
B cell markers play a crucial role in clinical settings for diagnosing and monitoring various diseases, including autoimmune disorders and lymphomas. Here is a discussion of the clinical applications of B cell markers in these contexts:
Autoimmune Disorders
B cell markers are widely used in the diagnosis and monitoring of autoimmune disorders, where B cells often play a central role in disease pathogenesis. Some common applications include:
- Anti-B cell therapy: B cell depletion therapy, such as rituximab, targets CD20-expressing B cells and is used in the treatment of autoimmune diseases like rheumatoid arthritis, systemic lupus erythematosus (SLE), and multiple sclerosis.
- Anti-B cell autoantibodies: Detection of autoantibodies produced by B cells, such as anti-nuclear antibodies (ANA), anti-double-stranded DNA (anti-dsDNA) antibodies, and anti-cyclic citrullinated peptide (anti-CCP) antibodies, helps in diagnosing autoimmune disorders like SLE and rheumatoid arthritis.
- B cell subset analysis: Flow cytometry-based analysis of B cell subsets, such as memory B cells and plasmablasts, can provide insights into disease activity and treatment response in autoimmune disorders.
Lymphomas
B cell markers are essential for the classification and diagnosis of various types of lymphomas, which are malignancies arising from B cells. Some key applications include:
- Immunophenotyping: Flow cytometry analysis of B cell markers, including CD19, CD20, CD22, and CD79a, helps differentiate B cell lymphomas from other types of lymphomas and determine their lineage and maturation stage.
- Molecular profiling: Determining the presence of specific B cell receptor rearrangements or gene mutations (e.g., MYD88, CD79B) can aid in the diagnosis and subclassification of B cell lymphomas.
- Minimal residual disease (MRD) monitoring: Monitoring B cell markers and clonal immunoglobulin gene rearrangements through quantitative PCR or next-generation sequencing can assess treatment response and detect minimal residual disease in patients with B cell lymphomas.
Other Applications
- Allergy and immunodeficiency: B cell markers, including surface immunoglobulin and CD27, are used in the diagnosis of primary immunodeficiencies and allergies.
- Infectious diseases: B cell markers can help assess immune responses to infections, such as monitoring B cell subset alterations during HIV infection or identifying specific B cell markers associated with response to vaccines.
In summary, B cell markers have extensive clinical applications in diagnosing, monitoring, and managing various diseases, including autoimmune disorders and lymphomas. They aid in the identification of disease-specific biomarkers, selection of targeted therapies, evaluation of treatment response, and monitoring of disease progression. The use of B cell markers in clinical practice continues to advance our understanding and management of these complex conditions.
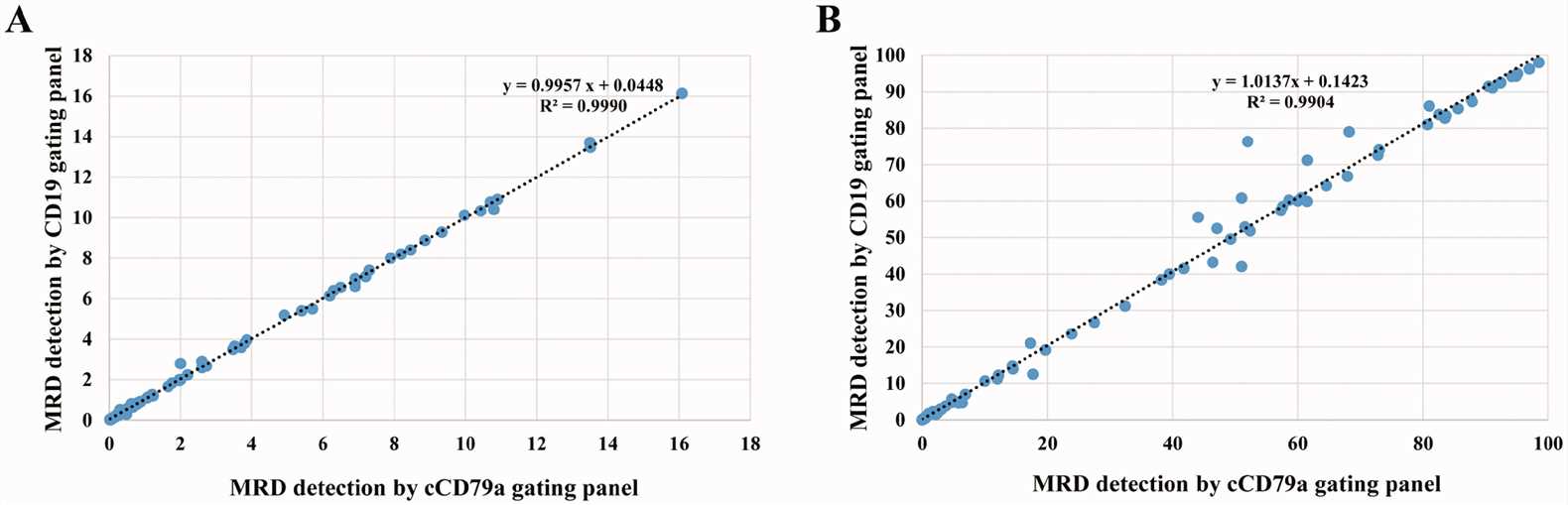 Fig.2 Linearity between the classical and cCD79a gating panels for detecting minimal residual disease (MRD). (Chen M, et al., 2022)
Fig.2 Linearity between the classical and cCD79a gating panels for detecting minimal residual disease (MRD). (Chen M, et al., 2022)Challenges and Limitations of B Cell Markers
While B cell markers are valuable tools in research and clinical settings, there are several challenges and limitations associated with their use. Here are some of the main challenges and limitations of B cell markers:
Variability in Marker Expression
- Heterogeneity within B cell populations: B cells exhibit significant heterogeneity in their marker expression profiles, which can vary depending on their differentiation stage, activation status, and tissue localization. This heterogeneity makes it challenging to define universal markers that accurately represent all B cells.
- Inter-individual variability: There can be variability in the expression levels of B cell markers among different individuals, which can complicate the interpretation of marker-based assays and comparisons between patients.
- Dynamic marker expression: B cell markers can be modulated in response to various stimuli, including infections, immune activation, and therapeutic interventions. This dynamic nature of marker expression adds complexity to their interpretation and monitoring over time.
Lack of Specificity
- Cross-reactivity with other cell types: Some B cell markers may also be expressed on other cell types, leading to potential cross-reactivity and difficulty in accurately identifying B cells without additional markers or techniques.
- Overlapping marker expression: The expression of certain markers may overlap between B cell subsets or with other immune cell populations, making it challenging to distinguish specific B cell subsets accurately.
Technical Limitations
- Detection sensitivity: Some B cell markers may be expressed at low levels, requiring sensitive detection methods to reliably identify and quantify them. This can be particularly relevant when studying rare B cell populations or minimal residual disease.
- Sample processing and preservation: The choice of sample processing and preservation methods can impact the integrity and expression of B cell markers, potentially introducing variability and affecting the accuracy of results.
- Compatibility with high-throughput techniques: Not all B cell markers are compatible with high-throughput techniques, such as single-cell RNA sequencing or mass cytometry, limiting their use in comprehensive profiling studies.
Limited Functional Insights
- Marker expression does not fully capture B cell functional diversity: B cell markers primarily provide information about the surface phenotype and developmental stage of B cells. Additional functional assays, such as cytokine production or proliferation assays, are often required to gain comprehensive insights into B cell functionality.
- Functional heterogeneity within marker-defined subsets: Even within marker-defined B cell subsets, there can be functional heterogeneity, with subsets exhibiting distinct functional properties or responses to stimuli. This heterogeneity may not be fully captured by marker-based analysis alone.
Addressing these challenges and limitations requires a comprehensive approach combining multiple markers, functional assays, and advanced technologies to enhance the accuracy and depth of B cell characterization. Researchers and clinicians need to carefully consider these factors when interpreting results and designing studies involving B cell markers.
Case Study
Case 1: Amu S, Lavy-Shahaf G, Cagigi A, et al. Frequency and phenotype of B cell subpopulations in young and aged HIV-1 infected patients receiving ART. Retrovirology. 2014;11:76.
The study examined the impact of HIV-1 infection on B cell phenotype, specifically focusing on the expression of inhibitory receptors (PD1 and FcRL4) and activation markers (CD25 and CD69). In young individuals, no significant differences were found in PD1 or FcRL4 expression among control, non-treated HIV-1 infected, and treated HIV-1 infected groups. However, CD25 expression was significantly reduced on certain B cell subsets in non-treated HIV-1 infected individuals compared to controls, and this reduction persisted even with treatment. CD69 expression on B cell subsets showed no significant differences between the groups. In the aged group, non-treated HIV-1 infected patients had lower CD25 expression on B cell subsets compared to controls, and treatment did not restore these differences. Additionally, CD69 expression was lower in all B cell subsets of non-treated aged patients compared to controls, and ART did not correct this. Overall, CD25 expression was similarly low in both young and aged HIV-1 infected individuals and was not corrected by treatment, while the difference in CD69 expression between aged controls and HIV-1 infected patients appeared to be influenced by increased expression in aged healthy controls rather than the virus's effect on B cells in infected patients.
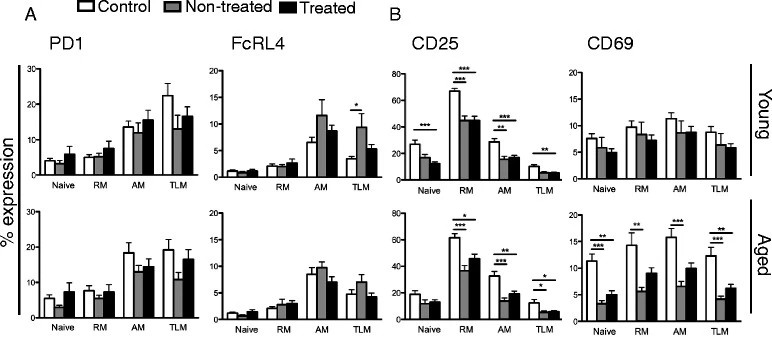 Fig.1 Exhaustion and activation markers on B cell subsets.
Fig.1 Exhaustion and activation markers on B cell subsets.Case 2: Königs C, Schultze-Strasser S, Quaiser A, et al. An exponential regression model reveals the continuous development of B cell subpopulations used as reference values in children. Front Pediatr. 2018;6:121.
The study examined B cell subpopulations in peripheral blood, including naïve, non-switched memory, and switched memory B cells. The naïve B cell population decreased in the first 10 years and remained stable until age 18, while non-switched, switched, and memory B cells increased within the first 5 years and remained stable thereafter. The absolute cell counts of these B cell subpopulations peaked at age 2 and then gradually decreased. The study also compared CD19 and CD20 as B cell markers and found no significant difference in total B cell numbers. However, the relative frequency of naïve B cells was slightly lower when using CD19, while the relative frequency of switched memory B cells was slightly higher.
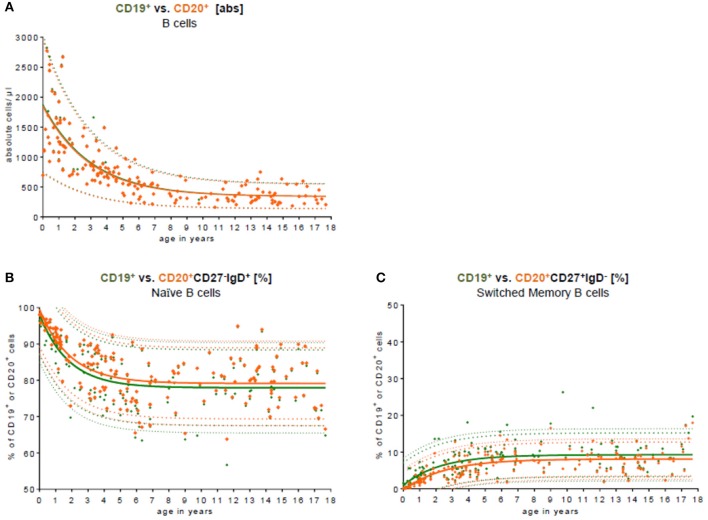 Fig.2 Comparison of CD19 and CD20 as key B cell markers.
Fig.2 Comparison of CD19 and CD20 as key B cell markers.
Reference
- Schett G, Nagy G, Krönke G, et alB-cell depletion in autoimmune diseases annals of the rheumatic diseases Published Online First: 22 May 2024. Chen M, Fu M, Wang A, et al. Cytoplasmic CD79a is a promising biomarker for B lymphoblastic leukemia follow up post CD19 CAR-T therapy. Leuk Lymphoma. 2022;63(2):426-434.

