Cytokines in Tumorigenesis
Related Symbol Search List
Immunology Background
Cytokine
Cytokines are a kind of small molecular proteins with extensive biological activities that are synthesized and secreted by immune cells (such as monocytes, macrophages, T cells, B cells, NK cells, etc.) and some non-immune cells (endothelial cells, epidermal cells, fibroblasts, etc.) after stimulation.
Cytokines generally regulate cell growth, differentiation and effects by binding to corresponding receptors and regulate the immune response. Cytokine (CK) is a low-molecularweight soluble protein induced by immunogen, mitogen or other stimulants in a variety of cells, and is capable of regulating innate and adaptive immunity, hemocyte production, cell growth, APSC pluripotent cells and repair of damaged tissues.
 Fig 1. Cytokine image
Fig 1. Cytokine image
The types of cytokines include interleukin, interferon, tumor necrosis factor, colony stimulating factor, chemotactic cytokines, transformed growth factor and growth factor.
-
Interleukin (IL) was named in 1979. Cytokines produced by lymphocytes, monocytes or other non-mononuclear cells play an important role in intercellular interaction, immune regulation, hematopoietic and inflammatory processes. CDNA gene cloning and expression of the named interleukin have been successful, and more than 30 species have been reported (IL-1- IL-38).
-
Interferon (IFN), is a cytokine discovered in 1957. It got its name from the discovery that cells infected with one virus could produce substances that interfered with the infection and replication of another. Depending on the source and structure of interferon production, ifn-alpha, ifn-beta, and ifn-gamma are produced by white blood cells, fibroblasts, and activated T cells, respectively. Different IFN biological activities are basically the same, with antiviral, anti-tumor and immunomodulatory effects.
-
Tumor necrosis factor (TNF) was first discovered to cause necrosis in tumor tissue. Depending on its origin and structure, tnf-alpha is produced by mononuclear macrophages, and tnf-beta is produced by activated T cells, also known as lymphophotoxin (LT). The basic biological activities of the two TNF types are similar. In addition to killing tumor cells, they also regulate the immune system and participate in the occurrence of fever and inflammation. High doses of TNF-α can cause dyscrasia, so TNF-α is also called cachectin.
-
Colony stimulating factor (CSF) according to different cytokines stimulate hematopoietic stem cells or hematopoietic cells of different differentiation stages to form different colonies in semi-solid medium. They were named G (granulocyte) -CSF, M (macrophage) -CSF, GM (granulocyte,Macrophages) -CSF, Multi (multiple) -CSF (IL-3), SCF, EPO, etc. Different CSF can not only stimulate the proliferation and differentiation of hematopoietic stem cells and progenitor cells at different stages of development, but also promote the function of mature cells.
-
The chemokine family includes four subfamilies:
1) CXC/α subfamily, mainly chemotactic neutrophils, the main members are IL-8, melanoma cell growth stimulating activity (GRO/MGSA), platelet factor-4 (PF-4), platelet alkali Sex protein, proteolytic derived products CTAP-III and β-thromboglobulin, inflammatory protein 10 (IP-10), ENA-78.
2) CC/β subfamily, a major chemotactic monocyte, members of this subfamily including macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES, monocyte chemoattractant protein-1 (MCP-1/ MCAF), MCP-2, MCP-3 and I-309.
3) Representative of the C-type subfamily is a lymphocyte chemotactic protein.
4) The CX3C subfamily, Fractalkine, is a CX3C chemokine that has chemotactic effects on monocyte-macrophages, T cells, and NK cells.
5) The transforming growth factor-β family (TGF-β family) is produced by a variety of cells, including TGF-β1, TGF-β2, TGF-β3, TGFβ1β2, and bone morphogenetic protein (BMP).
6) Growth factors (GF) such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and hepatocyte growth factor (HGF).
Cytokine Receptor
Cytokines function biologically by binding to corresponding cytokine receptors on the cell surface. The binding of cytokines to their receptors is the initiation stimulation of cytokine mediated cell signal transduction. Most of the known cytokine receptors are transmembrane proteins, which are composed of extracellular, transmembrane and cytoplasmic regions. The extracellular region recognizes the site of binding cytokines, and the cytoplasmic region initiates signal transduction after receptor activation.
Cytokine receptors can be divided into different families or superfamilies according to their structure and signal transduction pathway, such as type I cytokine receptors, type II cytokine receptors, tumor necrosis factor receptors and chemotactic cytokine receptors.
The class I cytokine receptor family includes IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-13, IL-15, GM-CSF and a receptor for cytokines such as erythropoietin. The class II cytokine receptor includes receptors for IFNα, IFNβ, IFNγ and IL-10. The tumor necrosis factor receptor superfamily (TNFSF) includes TNF receptor, nerve growth factor receptor, CD40 molecule and Fas molecule. The chemokinetic receptor family is a G-protein coupled receptor, a 7-transmembrane protein that binds to the corresponding ligand and exerts a biological effect by coupling a GTP-binding protein.
The cytokine receptor family performs a wide variety of essential biological functions including the growth and development of multiple cell types (e.g., blood cells) or regulation of the immune system. The diverse array of biological processes regulated by the cytokine receptors make them targets for disease-causing mutation or other modulation resulting in pathology. Mutations can cause gain-of-function or loss-of-function, leading to physiological states that may be characterized as being within normal limits or pathologic, depending on severity. Interestingly, each receptor does not seem to have an equal chance of acquiring a pathologic mutation. For instance, the Tpo receptor has several described mutations, but the Epo receptor does not. This may reflect a difference in the structural requirements for signaling, a difference in tolerance of variability in the ultimate physiological response, or differences in importance of the pathway either overall or at different stages of organism development. The most common mutation associated with increased cytokine receptor function occurs in the associated Janus kinase, in EpoR/TpoR, Jak2 (JAKV617F).
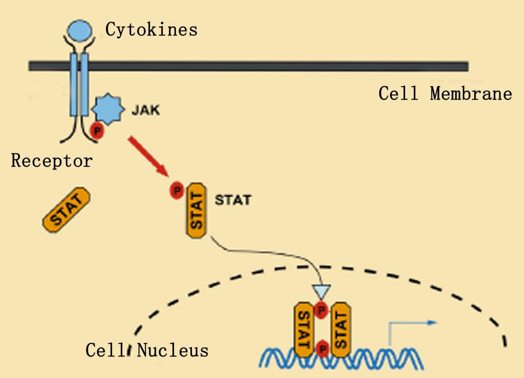 Fig.2 Enhancement of cytokine receptor function
Fig.2 Enhancement of cytokine receptor function
References:
1. Ihle J N. Cytokine receptor signalling[J]. Nature, 1995, 377(6550): 591.
2. Kishimoto T, Taga T, Akira S. Cytokine signal transduction[J]. Cell, 1994, 76(2): 253-262.
3. Starr R, Willson T A, Viney E M, et al. A family of cytokine-inducible inhibitors of signalling[J]. Nature, 1997, 387(6636): 917.

