MHC Class I
Related Symbol Search List
- LILRB1
- ILT4
- LILRB3
- LILRA1
- CD4
- ERAP1
- ERAP2
- FCGRT
- HLA-A
- HLA-C
- HLA-E
- KLRC3
- LILRB5
- MR1
- TAOK3
- TAPBP
- TAPBPL
Immunology Background
Background
About MHC Class I
Major Histocompatibility Complex (MHC) class I molecules are a group of cell surface proteins that play a crucial role in the immune system. They are found on the surface of almost all nucleated cells in the body, including cells of the immune system. MHC class I molecules are responsible for presenting antigens to cytotoxic CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), which are an essential component of the adaptive immune response.
The structure of MHC class I molecules consists of two main components:
- MHC Class I Heavy Chain
The heavy chain is a transmembrane protein that consists of three domains: α1, α2, and α3. The α1 and α2 domains form a peptide-binding groove where antigenic peptides are presented. These peptides are typically derived from intracellular proteins, such as viral or tumor antigens. The α3 domain interacts with other molecules, including CD8 co-receptor and inhibitory receptors.
- β2-Microglobulin
β2-microglobulin is a smaller protein that non-covalently associates with the MHC class I heavy chain. It is not directly involved in peptide binding but is necessary for the proper folding and stabilization of the MHC class I molecule. β2-microglobulin is encoded by a separate gene from the MHC, and its expression is required for the cell surface expression of MHC class I molecules.
The MHC class I molecule's primary function is to present peptide antigens derived from intracellular proteins to CD8+ T cells. This process is essential for the immune system to detect and eliminate infected or abnormal cells. The antigenic peptides are generated within the cells via the proteolytic degradation of proteins in the cytosol. These peptides bind to the peptide-binding groove of MHC class I molecules, forming stable MHC-peptide complexes.
The MHC class I-peptide complexes are then transported to the cell surface, where they are displayed for surveillance by CD8+ T cells. If a CD8+ T cell encounters a cell displaying a foreign or aberrant peptide on MHC class I, it can recognize and bind to the complex through its T cell receptor (TCR). This triggers the activation of the CD8+ T cell, leading to the release of cytotoxic molecules that can eliminate the infected or abnormal cell.
MHC class I molecules exhibit a high degree of polymorphism, meaning they can vary between individuals. This polymorphism allows the immune system to recognize a wide range of antigens, maximizing its ability to respond to diverse pathogens. MHC class I molecules are also involved in immune regulation, tolerance, and immune surveillance against tumors.
MHC Class I and Antigen Presentation
The process of MHC class I molecules binding to endogenous antigens and presenting them to T cells is known as antigen presentation. It plays a critical role in activating cytotoxic CD8+ T cells (CTLs) and initiating an immune response against infected or abnormal cells. Here's a step-by-step explanation of this process:
- Antigen Processing
Within the cytoplasm of cells, intracellular proteins, including viral proteins or abnormal self-proteins, are degraded by the proteasome, a cellular protein degradation machinery. This process generates short peptide fragments, typically 8-10 amino acids in length.
- Peptide Loading
The generated peptide fragments are transported into the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP) proteins. In the ER, the peptides bind to newly synthesized MHC class I molecules, which are already associated with β2-microglobulin.
- Peptide-MHC Class I Complex Formation
The binding of peptides to MHC class I molecules occurs within a groove formed by the α1 and α2 domains of the MHC class I heavy chain. The peptides that can stably bind to the groove are typically derived from proteins that are degraded within the cytoplasm.
- Quality Control
The peptide-MHC class I complexes undergo a quality control step to ensure that only properly formed complexes are transported to the cell surface. This quality control process occurs in the ER and involves interactions with chaperone molecules, such as calnexin and calreticulin, which assist in the proper folding and assembly of MHC class I molecules.
- Cell Surface Expression
The properly formed peptide-MHC class I complexes are transported from the ER to the cell surface via the Golgi apparatus. They are then displayed on the cell surface, where they can be recognized by CD8+ T cells.
- T Cell Recognition
CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), continuously survey the cell surface for peptide-MHC class I complexes. Each CD8+ T cell expresses a T cell receptor (TCR) that recognizes a specific peptide-MHC class I complex. When the TCR of a CD8+ T cell binds to a specific peptide-MHC class I complex that matches its receptor, it triggers a series of intracellular signaling events within the T cell.
- T Cell Activation
The binding of the TCR to the peptide-MHC class I complex, along with additional co-stimulatory signals, leads to the activation of the CD8+ T cell. This activation results in the expansion of the CD8+ T cell population, the production of effector molecules such as cytokines and cytotoxic granules, and the initiation of an immune response against the infected or abnormal cells.
By presenting endogenous antigens on their cell surface, MHC class I molecules provide a means for the immune system to detect and eliminate cells that are producing foreign or aberrant proteins, such as infected cells or cancer cells. This process is essential for immune surveillance, pathogen defense, and tumor immunity.
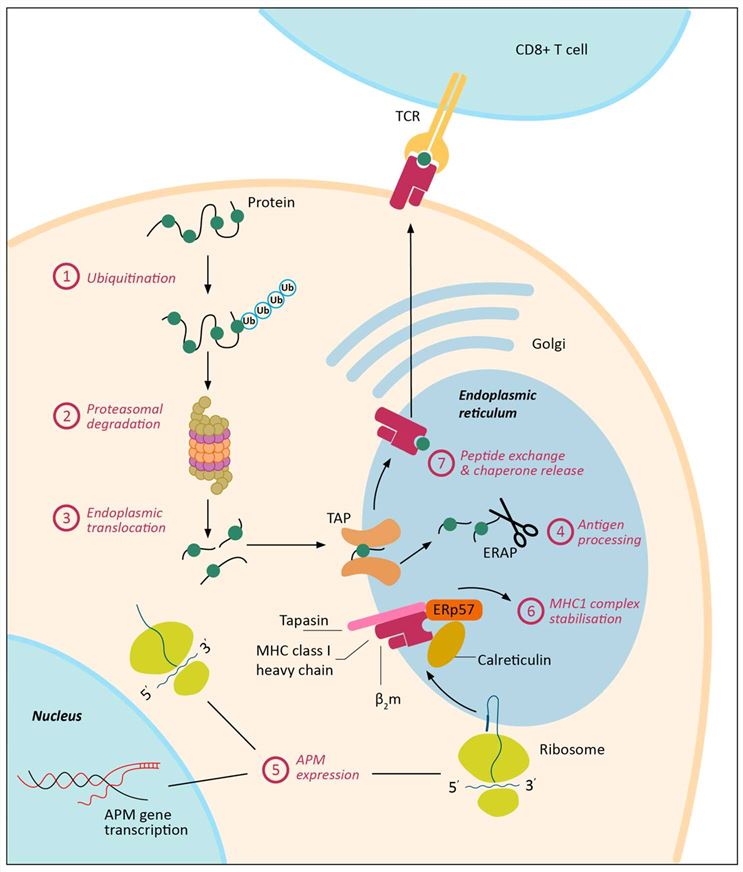 Fig.1 Major histocompatibility complex class I (MHC-I) antigen processing and presentation is a complex, multi-step process and can be dysregulated in cancer at multiple levels. (Cornel AM, et al., 2020)
Fig.1 Major histocompatibility complex class I (MHC-I) antigen processing and presentation is a complex, multi-step process and can be dysregulated in cancer at multiple levels. (Cornel AM, et al., 2020)Molecules of MHC Class I
Several MHC class I molecules play important roles in their assembly, transport, and function. Here are some key MHC class I molecules:
| Key molecule types | Functions |
|---|---|
| β2-Microglobulin (β2M) | β2-microglobulin is a small protein that non-covalently associates with the MHC class I heavy chain. It is essential for the proper folding, assembly, and stability of MHC class I molecules. β2M is encoded by a separate gene from the MHC genes and is required for the cell surface expression of MHC class I molecules. |
| Transporter Associated with Antigen Processing (TAP) | TAP is a heterodimeric protein complex consisting of TAP1 and TAP2 subunits. It is involved in transporting peptide antigens from the cytoplasm into the endoplasmic reticulum (ER), where they bind to MHC class I molecules. TAP plays a crucial role in the antigen processing and loading pathway of MHC class I molecules. |
| Chaperone Proteins | Several chaperone proteins assist in the proper folding, assembly, and quality control of MHC class I molecules. Calnexin and calreticulin are ER-resident chaperones that interact with MHC class I heavy chains during their maturation. They help ensure the proper folding and assembly of MHC class I molecules and promote the quality control of peptide loading. |
| Tapasin | Tapasin is an ER-resident protein that plays a critical role in the peptide loading process of MHC class I molecules. It interacts with both TAP and MHC class I molecules, bridging the gap between the transport of peptides by TAP and the assembly of peptide-MHC class I complexes. Tapasin helps facilitate the loading of high-affinity peptides onto MHC class I molecules. |
| ERAP (Endoplasmic Reticulum Aminopeptidase) | ERAP enzymes, including ERAP1 and ERAP2, are involved in trimming and editing the peptides that bind to MHC class I molecules. They process longer peptides generated by proteasomal degradation into shorter, optimal-length peptides that can bind efficiently to MHC class I molecules. ERAP enzymes contribute to the peptide repertoire presented by MHC class I molecules. |
| Peptide Loading Complex (PLC) | The peptide loading complex is a multi-molecular assembly involved in the proper assembly and quality control of peptide-MHC class I complexes. It consists of MHC class I molecules, tapasin, ERAP enzymes, chaperones, and other accessory proteins. The PLC coordinates the binding of peptides to MHC class I molecules, ensuring the proper conformation and stability of the complexes. |
These associated molecules, and others, are essential for the assembly, transport, quality control, and antigen presentation function of MHC class I molecules. They contribute to the generation of a diverse repertoire of peptide-MHC class I complexes, which are recognized by CD8+ T cells and initiate immune responses against infected or abnormal cells.
Regulation of MHC Class I in Health and Disease
Abnormalities or dysregulation in MHC class I molecules can have significant implications for the immune system, leading to various diseases and immunodeficiencies. Here are some examples of conditions associated with MHC class I abnormalities:
Autoimmune Diseases
Autoimmune diseases occur when the immune system mistakenly attacks the body's own cells and tissues. MHC class I molecules play a crucial role in presenting self-peptides to CD8+ T cells, which helps maintain immune tolerance and prevent the activation of autoreactive T cells. Abnormalities in MHC class I expression, such as reduced or altered expression levels, can disrupt self-tolerance mechanisms and contribute to the development of autoimmune diseases, including type 1 diabetes, rheumatoid arthritis, and psoriasis.
The HLA-Cw6 allele, a variant of MHC class I, is strongly associated with psoriasis susceptibility. Dysregulated antigen presentation by HLA-Cw6 may contribute to the activation of autoreactive T cells and the inflammatory processes underlying psoriasis.
Immunodeficiencies
Defects in MHC class I molecules or associated molecules can lead to immunodeficiencies characterized by impaired immune responses. For example, deficiencies in β2-microglobulin or TAP proteins can result in a condition known as bare lymphocyte syndrome (BLS). BLS patients lack cell surface expression of MHC class I molecules, leading to compromised CD8+ T cell responses and increased susceptibility to viral infections.
Viral Infections
Viruses have evolved strategies to evade the immune system, including interference with MHC class I antigen presentation. Some viral infections can downregulate MHC class I expression on infected cells or interfere with antigen processing and presentation pathways. This immune evasion mechanism allows viruses to evade recognition and destruction by CD8+ T cells. Examples include human immunodeficiency virus (HIV) and human cytomegalovirus (HCMV), which have developed strategies to impair MHC class I antigen presentation and evade immune responses.
Tumors
Abnormalities in MHC class I expression or antigen presentation pathways can have significant implications for tumor immunity. Reduced or absent MHC class I expression on tumor cells can limit their recognition and elimination by cytotoxic CD8+ T cells. Tumor cells may downregulate MHC class I molecules to evade immune surveillance and escape immune responses. This immune evasion mechanism is often associated with poor prognosis in cancer patients and can contribute to tumor progression and metastasis.
It's important to note that MHC class I abnormalities alone may not fully account for disease development or progression. They often interact with other genetic, environmental, and immunological factors to influence disease outcomes. Understanding the role of MHC class I abnormalities in different diseases is crucial for developing targeted therapies and immunomodulatory strategies to restore immune function and control immune-related disorders.
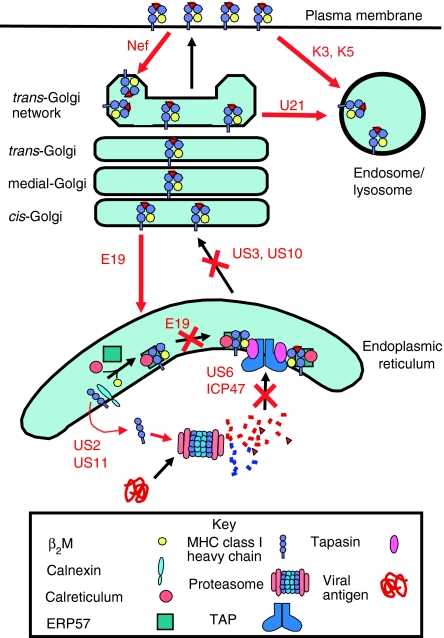 Fig.2 Viral inhibition of the MHC class I antigen presentation pathway. (Cornel AM, et al., 2020)
Fig.2 Viral inhibition of the MHC class I antigen presentation pathway. (Cornel AM, et al., 2020)Case Study
Case 1: Lerner EC, Woroniecka KI, D'Anniballe VM, et al. CD8+ T cells maintain killing of MHC-I-negative tumor cells through the NKG2D-NKG2DL axis. Nat Cancer. 2023;4(9):1258-1272.
The classically described mechanism of tumor immune escape is downregulation of tumor MHC-I. Here, we report that CD8+ T cells maintain the ability to kill tumor cells that completely lack MHC-I expression.
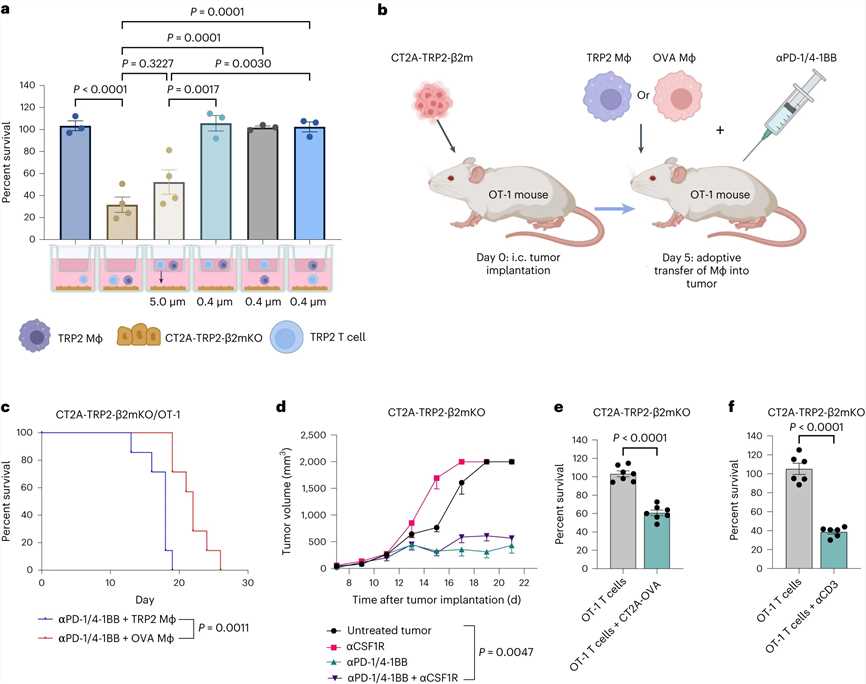 Fig.1 NKG2D on activated CD8+ T cells mediates killing of MHC-I-negative tumor cells in both murine and human tumor models.
Fig.1 NKG2D on activated CD8+ T cells mediates killing of MHC-I-negative tumor cells in both murine and human tumor models.Case 2: Cebrián C, Zucca FA, Mauri P, et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633.
Subsets of rodent neurons are reported to express major histocompatibility complex class I (MHC-I), but such expression has not been reported in normal adult human neurons. Here the authors provide evidence from immunolabeling, RNA expression, and mass spectrometry analyses of autopsy samples that human catecholaminergic substantia nigra and pallidum neurons express MHC-I and that this molecule can be induced in human stem cell-derived dopamine (DA) neurons.
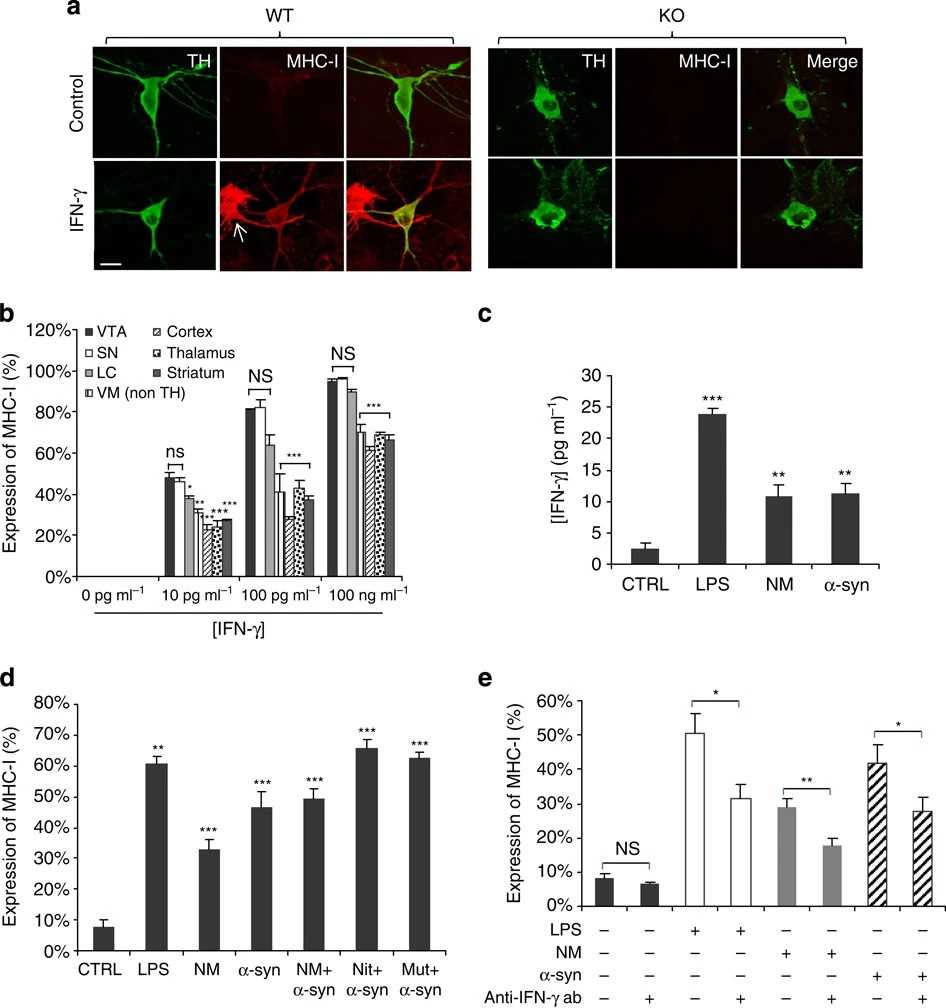 Fig.2 Induced MHC-I by murine catecholamine neurons.
Fig.2 Induced MHC-I by murine catecholamine neurons.References
- Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers. 2020; 12(7):1760.
- Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110(2):163-169.

