PLIN2
-
Official Full Name
perilipin 2 -
Overview
The protein encoded by this gene belongs to the perilipin family, members of which coat intracellular lipid storage droplets. This protein is associated with the lipid globule surface membrane material, and maybe involved in development and maintenance of adipose tissue. However, it is not restricted to adipocytes as previously thought, but is found in a wide range of cultured cell lines, including fibroblasts, endothelial and epithelial cells, and tissues, such as lactating mammary gland, adrenal cortex, Sertoli and Leydig cells, and hepatocytes in alcoholic liver cirrhosis, suggesting that it may serve as a marker of lipid accumulation in diverse cell types and diseases. Alternatively spliced transcript variants have been found for this gene. -
Synonyms
PLIN2;perilipin 2;ADFP;ADRP;adipose differentiation-related protein;adipophilin;Perilipin-2;Adipophilin;Adipose differentiation-related protein;perilipin-2;MGC1059;OTTHUMP00000021107
Recombinant Proteins
- Human
- Mouse
- Chicken
- Zebrafish
- E.coli
- Mammalian Cells
- HEK293
- In Vitro Cell Free System
- His
- T7
- Non
- Avi
- Fc
- Flag
- DDK
- Myc
- GST
Background
What is PLIN2 protein?
PLIN2 (perilipin 2) gene is a protein coding gene which situated on the short arm of chromosome 9 at locus 9p22. The protein encoded by this gene belongs to the perilipin family, members of which coat intracellular lipid storage droplets. This protein is associated with the lipid globule surface membrane material, and maybe involved in development and maintenance of adipose tissue. However, it is not restricted to adipocytes as previously thought, but is found in a wide range of cultured cell lines, including fibroblasts, endothelial and epithelial cells, and tissues, such as lactating mammary gland, adrenal cortex, Sertoli and Leydig cells, and hepatocytes in alcoholic liver cirrhosis, suggesting that it may serve as a marker of lipid accumulation in diverse cell types and diseases. The PLIN2 protein is consisted of 437 amino acids and its molecular mass is approximately 48.1 kDa.
What is the function of PLIN2 protein?
PLIN2 protein is a membrane protein in fat storage tissues that plays a key role in intracellular fat metabolism. One of the main functions of PLIN2 is to maintain the stability of lipid droplets, which it does by limiting the fusion of lipid droplets and promoting the decomposition of lipid droplets. In addition, PLIN2 is involved in regulating the transport and secretion of lipid droplets to ensure intracellular lipid balance. Abnormal expression of PLIN2 is associated with a variety of diseases, including obesity, diabetes, and nonalcoholic fatty liver disease.
PLIN2 Related Signaling Pathway
The main signaling pathways involved in PLIN2 include insulin signaling, fatty acid uptake and storage, and lipolysis. By interacting with the Hedgehog signaling pathway, PLIN2 regulates adipocyte differentiation and lipid storage. At the same time, it is also involved in the AMP-activated protein kinase (AMPK) signaling pathway, which responds to cellular energy state and regulates the oxidation of fatty acids. In an overtrophic state, PLIN2 expression is regulated by transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and carbohydrate response element binding protein (ChREBP), which play key roles in adipogenesis and glucose metabolism.
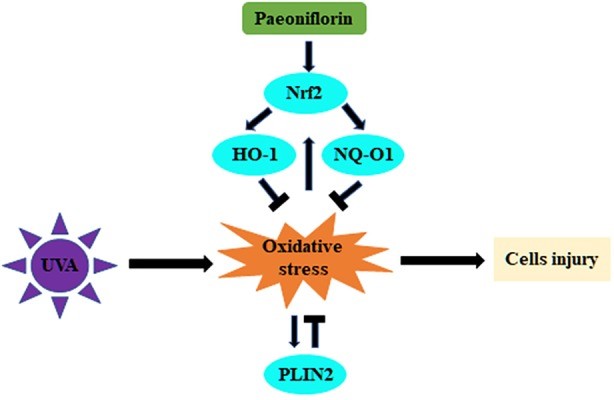
Fig1. Schematic illustration of this study showing the regulation of UVA-related oxidative stress by PF via Nrf2/HO-1/NQ-O1 signaling pathway or by PLIN2. (Yan-Song Lu, 2020)
PLIN2 Related Diseases
Diseases associated with PLIN2 include obesity, diabetes, non-alcoholic fatty liver disease, and certain types of cancer. In obesity, increased PLIN2 expression is associated with hypertrophy and dysfunction of fat cells. PLIN2 expression is also commonly upregulated in diabetic patients and may be associated with insulin resistance and hyperglycemia. The change of PLIN2 expression in patients with nonalcoholic fatty liver disease may be related to liver fat deposition and inflammatory response. In addition, the expression of PLIN2 also changes in some cancer types, which may be related to the proliferation, migration and invasion ability of tumor cells.
Bioapplications of PLIN2
In its mature applications, PLIN2 is mainly used to study fat metabolism and diseases, such as obesity and metabolic syndrome. Through its role in regulating lipid droplet production and fatty acid intake, we can deeply understand the function of fat cells and the pathogenesis of related diseases, and provide important references for the treatment and prevention of related diseases.
Case Study
Case study 1: Rong Hu, 2017
Natural antibodies used as biorecognition elements suffer from numerous shortcomings, such as limited chemical and environmental stability and cost. Artificial antibodies based on molecular imprinting are an attractive alternative to natural antibodies. The researchers investigated the role of aromatic interactions in target recognition capabilities of artificial antibodies. Three proteins with different aromatic amino acid content were employed as model targets. Artificial antibodies were formed on nanostructures using combinations of silane monomers of varying aromatic functionality. They employed refractive index sensitivity of plasmonic nanostructures as a transduction platform for monitoring various steps in the imprinting process and to quantify the target recognition capabilities of the artificial antibodies. The sensitivity of the artificial antibodies with aromatic interactions exhibited a protein-dependent enhancement. Selectivity and sensitivity enhancement due to the presence of aromatic groups in imprinted polymer matrix was found to be higher for target proteins with higher aromatic amino acid content. The results indicate that tailoring the monomer composition based on the amino acid content of the target protein can improve the sensitivity of plasmonic biosensors based on artificial antibodies without affecting the selectivity.
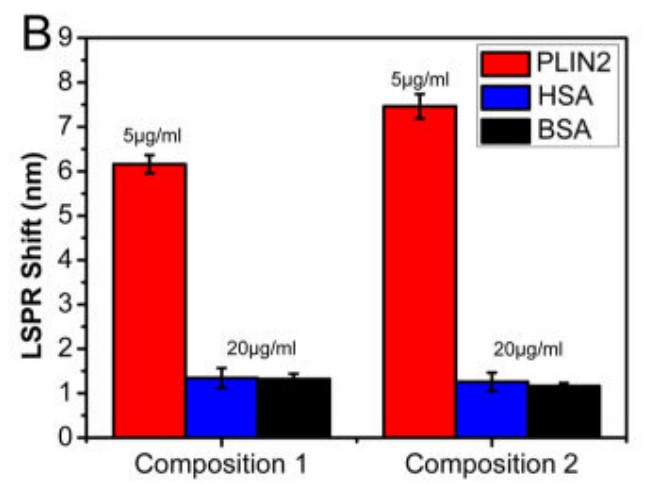
Fig1. Specificity of the PLIN2-imprinted artificial antibody compared with interference protein (HSA or BSA) at distinct concentrations with polymers of different compositions.
Case study 2: Xin Tong, 2021
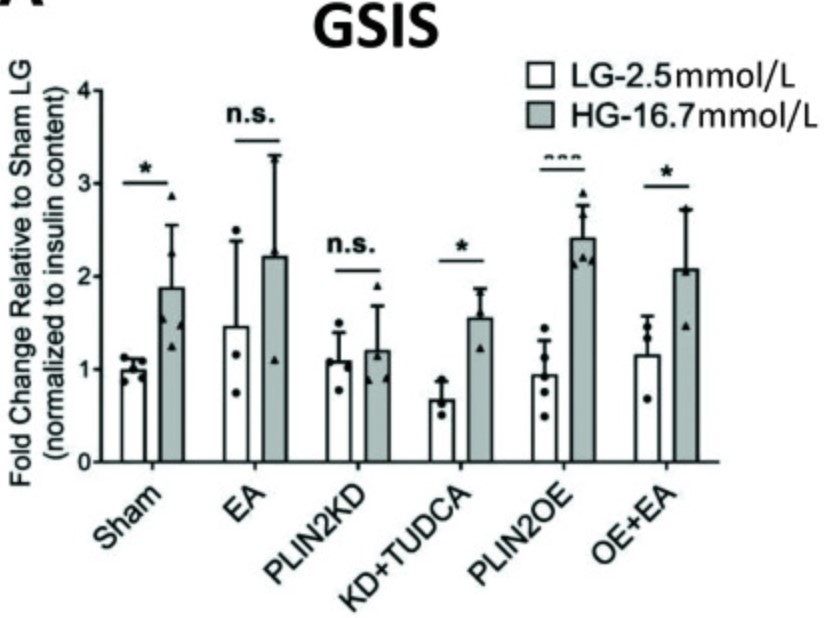
Free fatty acids (FFAs) are often stored in lipid droplet (LD) depots for eventual metabolic and/or synthetic use in many cell types, such a muscle, liver, and fat. In pancreatic islets, overt LD accumulation was detected in humans but not mice. LD buildup in islets was principally observed after roughly 11 years of age, increasing throughout adulthood under physiologic conditions, and also enriched in type 2 diabetes. To obtain insight into the role of LDs in human islet β-cell function, the levels of a key LD scaffold protein, perilipin 2 (PLIN2), were manipulated by lentiviral-mediated knockdown (KD) or overexpression (OE) in EndoCβH2-Cre cells, a human cell line with adult islet β-like properties. Glucose-stimulated insulin secretion was blunted in PLIN2KD cells and improved in PLIN2OE cells. An unbiased transcriptomic analysis revealed that limiting LD formation induced effectors of endoplasmic reticulum (ER) stress that compromised the expression of critical β-cell function and identity genes. These changes were essentially reversed by PLIN2OE or using the ER stress inhibitor, tauroursodeoxycholic acid. These results strongly suggest that LDs are essential for adult human islet β-cell activity by preserving FFA homeostasis.
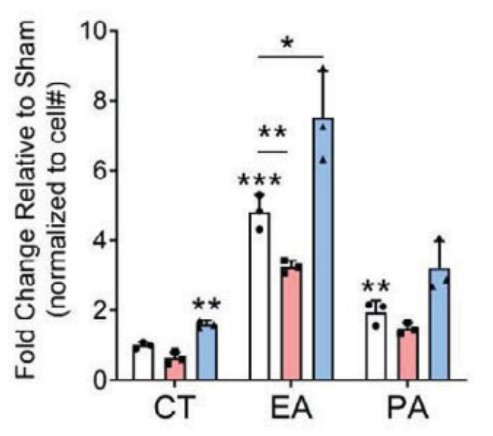
Fig3. Quantitation of the change in LD levels between Sham and PLIN2KD or PLIN2OE cells incubated without (control [CT]) or with 500 μmol/L EA or PA for 24 h.
Quality Guarantee
High Purity
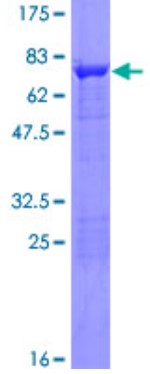
Fig1. SDS-PAGE (PLIN2-341H) (PROTOCOL for western blot)
.
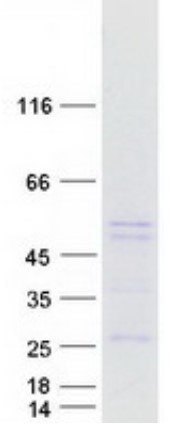
Fig2. SDS-PAGE (PLIN2-3014H) (PROTOCOL for western blot)
Involved Pathway
PLIN2 involved in several pathways and played different roles in them. We selected most pathways PLIN2 participated on our site, such as Adipogenesis,Fatty acid, triacylglycerol, and ketone body metabolism,HIF-1-alpha transcription factor network, which may be useful for your reference. Also, other proteins which involved in the same pathway with PLIN2 were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Regulation of lipid metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ANKRD1,MED21,CTGF,MED9,MED31,MED25,MED26,CDK19,MTF1,MED23 |
| Metabolism | Sult2a2,CYP2D6,OAZ2B,CPNE1,OAZ1A,ARV1,DHCR24,CHKB,GPHN,CYP2U1 |
| Metabolism of lipids and lipoproteins | ARF3,PMVK,CEPT1A,MED20,COQ7,ALOX5AP,AKR1C4,SLC25A1,ARSI,LPA |
| Fatty acid, triacylglycerol, and ketone body metabolism | THEM5,GLIPR1,MED9,CYP4A11,ELOVL1A,CPT1B,ACOT10,ACOT12,GPAT2,ACOT11 |
| PPARA activates gene expression | MED18,MED19,MED21,ANKRD1,MED6,MED9,GLIPR1,MED7,MED15,MED29 |
| HIF-1-alpha transcription factor network | NPM1,TFF3,ENG,ADM,CITED2,CP,COPS5,EGLN3,GATA2,NDRG1 |
| Adipogenesis | CISD1,GADD45A,DDIT3,BSCL2,E2F4,EPAS1B,MIXL1,BMP3,GDF10,AHR |
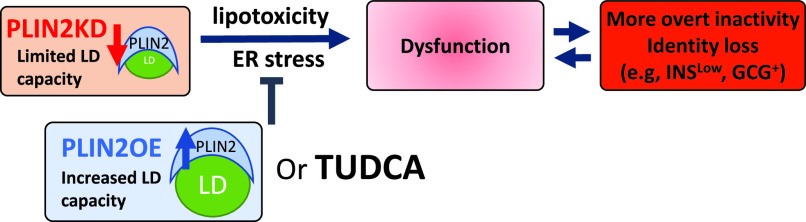
Fig1. Model illustrating the proposed importance of FFA storage in LDs to human β-cell health. (Xin Tong, 2021)
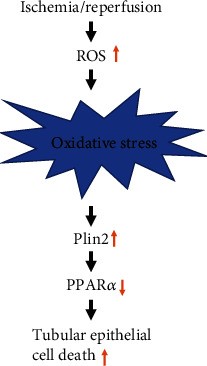
Fig2. Proposed schema of the pathway for I/R-induced AKI, involving the promotion of mitochondrial reactive oxygen species (ROS) generation, upregulation of Plin2, and downregulation of PPARα, resulting in cell apoptosis. (Sujuan Xu, 2021)
Protein Function
PLIN2 has several biochemical functions, for example, . Some of the functions are cooperated with other proteins, some of the functions could acted by PLIN2 itself. We selected most functions PLIN2 had, and list some proteins which have the same functions with PLIN2. You can find most of the proteins on our site.
| Function | Related Protein |
|---|
Interacting Protein
PLIN2 has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with PLIN2 here. Most of them are supplied by our site. Hope this information will be useful for your research of PLIN2.
MAPK10;ftsX
Resources
Research Area
Related Services
Related Products
References
- Kimura, A; Sakurai, T; et al. Identification of Target Antigens of Antiendothelial Cell Antibodies Against Human Brain Microvascular Endothelial Cells in Healthy Subjects. CURRENT NEUROVASCULAR RESEARCH 12:25-30(2015).
- Mulay, K; White, VA; et al. Sebaceous carcinoma: clinicopathologic features and diagnostic role of immunohistochemistry (including androgen receptor). CANADIAN JOURNAL OF OPHTHALMOLOGY-JOURNAL CANADIEN D OPHTALMOLOGIE 49:326-332(2014).




