TransTAC, a novel molecular archetype for membrane protein degradation in cancers and other cell types
Membrane proteins play critical roles in cellular signaling, nutrient transport, and disease progression. However, their degradation has remained challenging due to their localization and structural complexity. Targeted protein degradation (TPD) strategies, such as PROTACs and LYTACs, have emerged as promising tools but face limitations in tissue specificity and versatility. Transferrin Receptor Targeting Chimeras (TransTACs), a novel approach leveraging the transferrin receptor 1 (TfR1), which is overexpressed in cancer cells and activated immune cells, to degrade diverse membrane proteins with high efficiency and specificity.
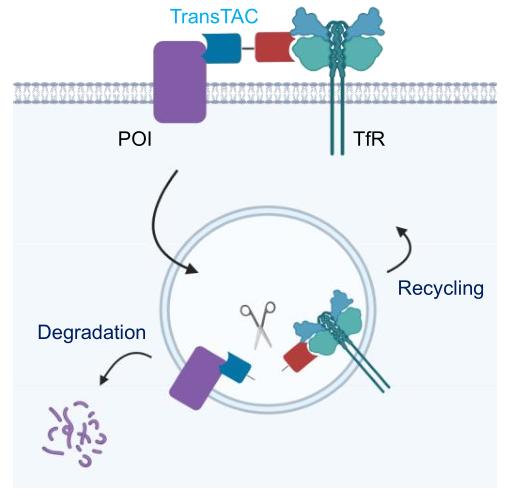
Key Innovations and Design Principles
- Rationale for TfR1 Utilization:
- TfR1 is upregulated in rapidly dividing cells, including cancers and activated T cells, due to their high iron demand.
- TfR1 exhibits rapid internalization (500 molecules/cell/second), enabling efficient cargo delivery to lysosomes.
- TransTAC Engineering:
- Dimerization: Dimeric TransTACs (e.g., v0.2) outperformed monomeric versions by enhancing avidity and reducing "hook effects."
- Cleavable Linkers: Incorporation of cathepsin-sensitive linkers (e.g., v0.4) ensured separation of the protein of interest (POI) from TfR1 in early endosomes, redirecting POIs to lysosomes.
- Antibody-Based Binding: Replacing transferrin ligands with anti-TfR1 scFv antibodies (e.g., v1.0) minimized recycling and improved degradation efficiency (>80% for CAR, PD-L1, EGFR, and CD20).
- Mechanistic Insights:
- Degradation relies on lysosomal pathways (blocked by bafilomycin) rather than proteasomes.
- TfR1 levels remain stable post-treatment, confirming POI-specific degradation.
Applications and Results
- Broad Target Range:
- Synthetic Receptors: CAR-TransTACs degraded chimeric antigen receptors (CARs) in T cells, offering a reversible "OFF switch" to mitigate cytokine release syndrome (IC50 = 0.4 nM).
- Native Proteins: PD-L1, EGFR, and CD20 were degraded in cancer cells (up to 98% efficiency), demonstrating applicability to single-pass (PD-L1) and multi-pass (CD20) transmembrane proteins.
- In Vivo Validation:
- CD20-TransTACs administered to mice showed favorable pharmacokinetics (half-life ~10 days) and no toxicity, supporting translational potential.
- Kinetics and Specificity:
- Rapid internalization (80% CAR removal within 10 minutes) and sustained effects highlight TransTACs as tools for dynamic protein regulation.
- Tumor specificity is enabled by TfR1 overexpression in cancers, minimizing off-target effects.
Therapeutic and Research Implications
- Cancer Therapy: TransTACs could degrade oncogenic receptors (e.g., EGFR) or immune checkpoints (e.g., PD-L1) to enhance anti-tumor responses.
- CAR-T Regulation: As a modular, non-genetic OFF switch, TransTACs may improve the safety of CAR-T therapies.
- Basic Research: The rapid kinetics enable temporal studies of membrane protein function in signaling and trafficking.
TransTACs represent a groundbreaking advancement in TPD, combining the natural recycling efficiency of TfR1 with engineered protein design to achieve potent, specific degradation of diverse membrane targets. Their modularity, tumor specificity, and rapid action position them as transformative tools for both therapeutic development and fundamental research. Future work will likely explore clinical translation, combination therapies, and expansion to additional disease contexts. This study bridges a critical gap in membrane protein degradation, offering a universal platform with implications for precision medicine, immunotherapy, and beyond.
Reference
- Zhang D, Duque-Jimenez J, Facchinetti F, Brixi G, Rhee K, Feng WW, Jänne PA, Zhou X. Transferrin receptor targeting chimeras for membrane protein degradation. Nature. 2025 Feb;638(8051):787-795. doi: 10.1038/s41586-024-07947-3. Epub 2024 Sep 25. PMID: 39322661; PMCID: PMC11839386.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

