Stem Cell Lysates
Background
Overview
Stem cell lysate usually refers to the mixture containing a variety of bioactive factors obtained after the stem cells are decomposed by specific methods (such as ultrasonic crushing, enzymatic hydrolysis, etc.) in the process of stem cell culture. These active factors include growth factors, cytokines, extracellular matrix proteins, etc., which play an important role in the growth, differentiation and function maintenance of other cells.
Production procedure
The production process of stem cell lysate involves several key steps to ensure the quality, safety and consistency of the final product. Here are some key steps:
Cell culture: First, stem cells, such as mesenchymal stem cells (MSCs), need to be grown under suitable conditions. This includes selecting the right medium, cell density, temperature, and CO2 levels.
Cell collection: After the cells have reached the appropriate growth stage, the cells need to be collected. This usually involves washing the cells with cold PBS or other buffers to remove impurities from the medium.
Cleavage: The collected cells are then treated with a lysate, which may contain surfactants such as Triton X-100, as well as possible protease inhibitors such as PMSF, to prevent the activity of proteases within the cell from causing protein degradation.
Lysate collection: After lysate, cell contents are released into lysate to form lysate. This step is needed to ensure that the cells are sufficiently cleaved to collect enough proteins and other bioactive molecules.
Centrifugation and clarification: Lysates usually need to be centrifuged to remove unlysated cell fragments and larger cell remains to obtain a clear lysate supernatant.
Protein concentration determination: In order to ensure consistency and sufficient concentration of protein in the lysate, it is necessary to determine protein concentration using methods such as Bradford or BCA protein quantification.
Quality control: Throughout the production process, quality control testing is required, including sterility testing, endotoxin testing, protein electrophoresis analysis, etc., to ensure the safety and biological activity of the lysate.
Lysate Compositions
Growth factors: such as epidermal growth factor (EGF), fibroblast growth factor (FGF), nerve growth factor (NGF), platelet-derived growth factor (PDGF), etc. These growth factors play an important role in cell proliferation, migration and differentiation.
Cytokines: such as interleukin (IL), tumor necrosis factor (TNF), etc., these proteins play a role in regulating the immune response and inflammatory response.
Hormones: such as insulin-like growth factor (IGF), transforming growth factor-β (TGF-β), etc., which are involved in regulating metabolism and cell fate determination.
Nucleic acid fragments: includes DNA and RNA fragments, which may contain gene sequences encoding various proteins and RNA molecules such as microRNA.
Proteins: In addition to the growth factors and cytokines mentioned above, there may also be other types of proteins, such as structural proteins, enzymes, etc.
Metabolites: such as amino acids, sugars, lipids, etc., which are direct products of cell physiological activities.
Research Progress
The research progress of stem cell lysates changes with the development of stem cell research, which is mainly divided into three stages.
The exploration of therapeutic potential. Stem cells have the characteristics of self-renewal and differentiation potential, which makes them have great application prospects in tissue regeneration and repair. Current research covers many types of stem cells, including hematopoietic stem cells, epidermal stem cells, muscle stem cells, mesenchymal stem cells, and pluripotent stem cells for nerve regeneration.
Clinical study phase. Worldwide, a major portion of stem cell clinical research is in the early stages, with 20.4% in phase I or early, while confirmative phase III clinical studies account for only 8.3%.
Applications in cancer therapy. Cancer stem cells, as a subgroup of tumor cells, have attracted much attention due to their enhanced ability to self-renew, metastasize and spread, and treatment resistance. Current research is exploring the origin of cancer stem cells and their role in tumorigenesis, recurrence and metastasis, which has important implications for the development of new cancer treatments.
Advantages
Animal-free: Some stem cell lysates, such as human platelet lysates (HPL), can be used as animal-free culture supplements, which is important to avoid potential animal-derived disease transmission and ethical concerns.
Safety: Under serum-free or low-serum culture conditions, the use of stem cell lysates can reduce the use of animal-derived components, thereby reducing the potential risk of pathogen contamination during culture and improving the safety of cell products.
Applications
- Tissue repair and regeneration: After tissue injury, stem cell lysates can be used to promote the repair and regeneration of damaged tissue. For example, in cases of skin injury, myocardial infarction, or nerve damage, factors in stem cell lysates can stimulate the activation and proliferation of endogenous stem cells, thereby accelerating wound healing and tissue reconstruction.
- Cell culture additives: In vitro cell culture, stem cell lysate can be used as a serum substitute to provide nutrients and growth factors required for cell growth, especially in serum-free or low-serum culture conditions, to help maintain the pluripotent stem cells and promote the differentiation of specific cell types.
- Immune regulation: Some stem cell lysates contain factors that regulate the immune system and can be used to treat autoimmune diseases. For example, anti-inflammatory factors in the lysates of mesenchymal stem cells (MSCs) can reduce the inflammatory response, and MSCs have been used in regenerative medicine to treat a variety of autoimmune diseases.
- Gene therapy: In gene therapy, stem cell lysates can act as vectors to help deliver therapeutic genes into target cells. In addition, certain factors in the lysate may have an impact on the efficiency and safety of the gene editing technology, so these factors need to be considered when designing gene therapy strategies.
- 3D bioprinting: In 3D bioprinting, stem cell lysates can be used as part of a bio-ink, providing the environment necessary for cell growth and helping to build biologically active tissue structures.
Case Study
Case Study 1: Umbilical Cord Stem Cell Lysate
This study aims to compare in vivo, the acute anti-inflammatory effects of a lysate derived from human umbilical perivascular mesenchymal cells with the cells themselves in both an established hind-paw model of carrageenan-induced inflammation and also in the inflamed temporomandibular joint. Human umbilical cord perivascular cells were harvested and cultured in xeno- and serum-free conditions to P3. In addition, P3 cells were used to prepare a proprietary 0.22 micron filtered lysate. First, CD1 immunocompetent mice underwent unilateral hind-paw injections of carrageenan for induction of inflammation, followed immediately by treatment with saline (negative control), 1% cell lysate, or viable cells. Second, immunocompetent Male Wistar rats underwent unilateral intra-articular temporomandibular (TMJ) injections from the same treatment groups and were sacrificed at 4 and 48 hr post-injection. The lysate and cell-treated hind-paw demonstrated reduced tissue edema, and significantly lower concentrations of myeloperoxidase and TNF-alpha at 48 hr compared to untreated controls.
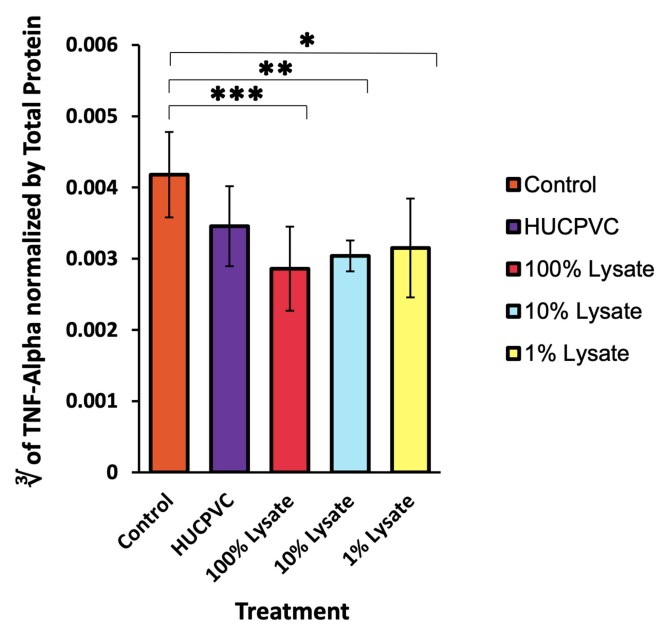
(Christopher K Ward, 2023)
Fig1. TNF-alpha levels were measured 48 hours post-induction of inflammation and normalized using total protein.
Case Study 2: Human Embryonic Stem Cells Lysate
Glycosylation, especially N-glycosylation, is one of the most common protein modifications. However, due to the complexity of the cellular glycosylation process, in-depth glycoanalysis is still a highly challenging endeavor. Contamination of samples with oligosaccharide impurities (OSIs), typically linear glucose homo-oligomers, can cause further complications. However, removing it is a time-consuming and labor-intensive task. Thus this study investigated the option to enzymatically degrade and thereby remove interfering OSIs as a final sample preparation step. Therefore, ten commercially available enzymes were screened concerning their potential to efficiently degrade maltodextrins and dextrans as most frequently found OSIs. Of these enzymes, only dextranase from Chaetomium erraticum and glucoamylase P from Hormoconis resinae enabled a degradation of OSIs within only 30 min that is free of side reactions with N-glycans. Finally, they applied the straightforward enzymatic degradation of OSIs to N-glycan samples derived from different standard glycoproteins and various stem cell lysates.
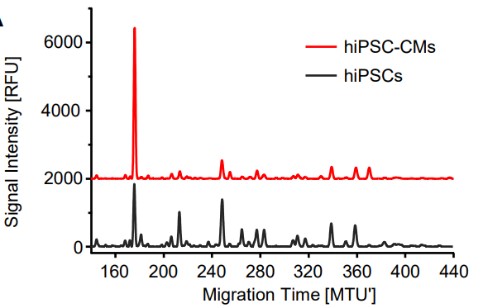
(Robert Burock, 2023)
Fig2. N-glycans of human-induced pluripotent stem cells (hiPSCs) and stem cell-derived cardiomyocytes (hiPSC-CMs) were analyzed.
Case Study 3: Mesenchymal Stem Cells Lysate
Acute liver injury (ALI) induced by sepsis seriously endangers the health of human beings every year. Mesenchymal stem cells (MSCs) lysate containing various regulators had a positive effect on anti-inflammation, hoping to provide a promising strategy in ALI. Olfactory mucosa-derived mesenchymal stem cells (OM-MSCs) were extracted and identified. The components of OM-MSCs lysate were detected by western blotting. In vitro, OM-MSCs lysate was applied to evaluate the effects on normal human liver cells (LO-2) under stimulation of LPS. Lipopolysaccharide (LPS) was also injected intraperitoneally to build ALI model in mice. We further assessed the anti-inflammatory capacity of OM-MSCs lysate on ALI in vivo by aminotransferase determination, pathology observation, and immunohistochemical staining. Moreover, the immunoblot technique was performed to recognize the changes in inflammatory factors and related proteins. OM-MSCs lysate could protect structure effectively. A significant decrease in tumor necrosis factor-α (TNF-α) also occurred under the treatment of OM-MSCs lysate. Up-expression of vascular endothelial growth factor (VEGF) in vivo revealed that OM-MSCs might have a great potential for healing.
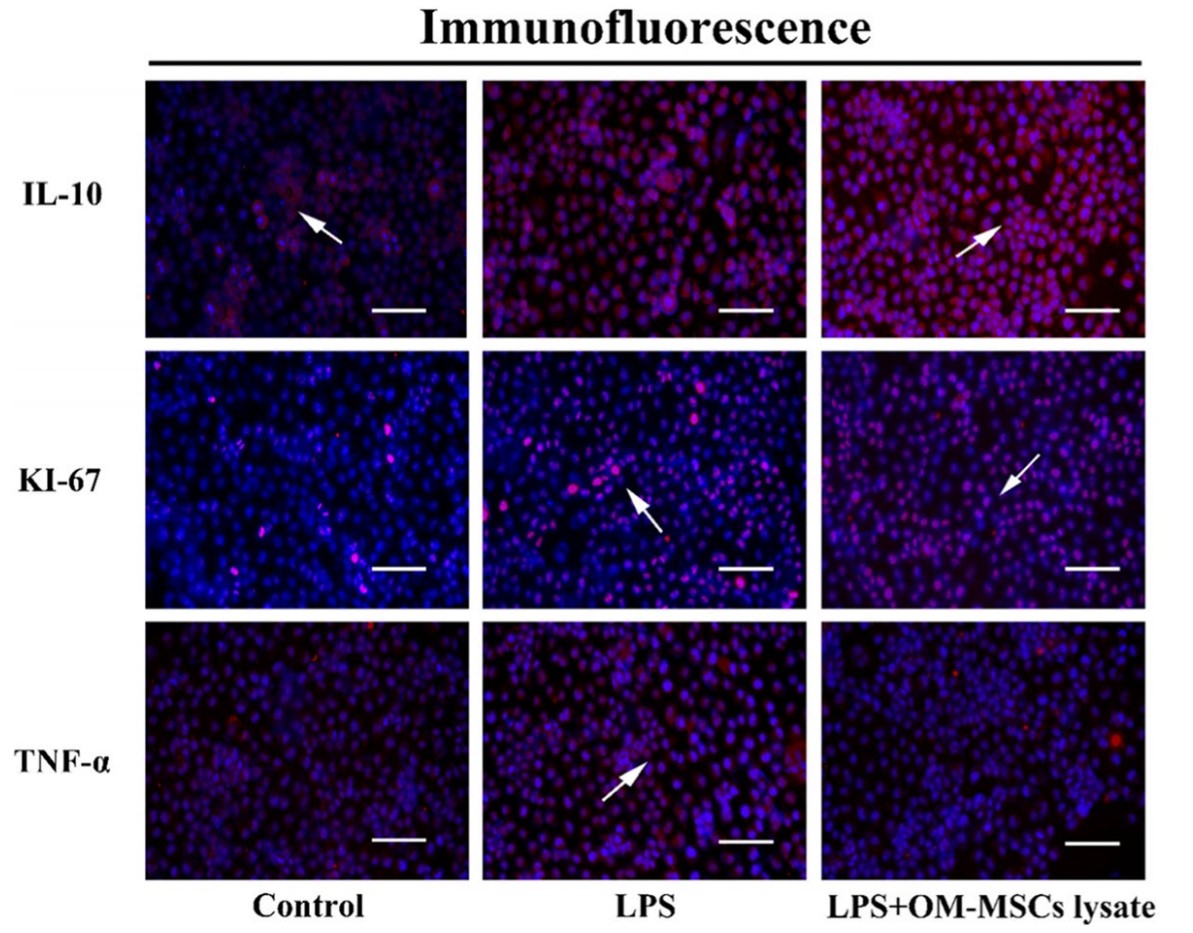
(Zhe Wang, 2022)
Fig3. Evaluation of anti-inflammation and proliferative abilities of OM-MSCs lysate in vitro.
Advantages
- Pure and efficient. We use advanced biotechnology to ensure unparalleled purity and activity in each batch, making your test results more accurate and reliable.
- Technical support. Purchase our products and you will receive full technical support. Our team of scientists is ready to answer your questions and help you break through your research bottlenecks.
- Reputation Guarantee. We promise that all products are subject to strict quality control, and guarantee fast and safe logistics distribution, so that your research results are not delayed due to supply problems.
- Strong scientific research strength. Our research team has a rich academic background and we are always exploring more popular products.
Creative BioMart can provide various types of Stem Cell Lysates to meet different research goals. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- Ward CK.; et al. Umbilical Cord Stem Cell Lysate: A New Biologic Injectate for the Putative Treatment of Acute Temporomandibular Joint Inflammation. J Inflamm Res. 2023;16:4287-4300.
- Burock R.; et al. Reliable N-Glycan Analysis-Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation. Molecules. 2023;28(4):1843.
- Wang Z.; et al. Olfactory mucosa tissue-derived mesenchymal stem cells lysate ameliorates LPS-induced acute liver injury in mice. BMC Pulm Med. 2022;22(1):414.





