Interferon
Related Symbol Search List
Immunology Background
Available Resources for Interferon Research
At Creative BioMart, we understand the importance of comprehensive support for researchers in their studies of interferons. Therefore, we offer a wide variety of carefully developed products to meet their specific research needs. Our product range includes recombinant proteins, protein-pre-coupled magnetic beads, cell and tissue lysates, and more, ensuring that researchers have access to the tools necessary for their investigations across diverse fields.
In addition to our extensive product selection, we are dedicated to providing a wealth of knowledge on interferons. Our resources encompass essential topics including pathways, protein functions, interacting proteins, related articles, and research areas. These valuable insights serve as a valuable reference for researchers aiming to deepen their understanding of these molecules and their critical roles in various physiological processes.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| IFNA1-14074H | Recombinant Human IFNA1, GST-tagged | Human | E.coli | GST |
| IFNA10-619H | Recombinant Human IFNA10 Protein, His-tagged | Human | E.coli | His |
| Ifna13-618M | Recombinant Mouse Ifna13 Protein, His-tagged | Mouse | E.coli | His |
| IFNA2-01H | Recombinant Human IFNA2 protein | Human | Yeast | N/A |
| IFNA4-29H | Active Recombinant Human IFNA4 protein, hFc-tagged | Human | HEK293 | hFc |
| IFN-a-62B | Recombinant Bovine Interferon-Alpha | Bovine | Yeast | N/A |
| IFNA5-30H | Recombinant Human IFNA5, Fc tagged | Human | Human Cell | Fc |
| IFNA7-621H | Recombinant Human IFNA7 Protein, His-tagged | Human | E.coli | His |
| IFNB1-14H | Recombinant Human IFNB1, Fc tagged | Human | Human Cell | Fc |
| IFNG-510H | Active Recombinant Human IFNG | Human | HEK293 | N/A |
| IL29-381H | Recombinant Human IL29 protein, His-tagged | Human | HEK293 | His |
| IL28B-379H | Recombinant Human IL28B, His tagged | Human | Human Cell | His |
| IFNW1-32H | Active Recombinant Human IFNW1 protein, hFc-tagged | Human | HEK293 | hFc |
About Interferons
Interferons (IFNs) are a group of proteins that play a critical role in the body's immune response to viral infections, as well as in regulating immune cell functions. They are part of the cytokine family, which are small signaling molecules involved in cell-to-cell communication.
There are three main types of interferons: Type I (including IFN-alpha and IFN-beta), Type II (IFN-gamma), and Type III (IFN-lambda). Each type has distinct functions and is produced by different cell types in response to viral infections or other immune stimuli.
Interferons are primarily produced by infected cells, particularly in response to viral RNA or other pathogen-associated molecular patterns (PAMPs). Upon detection of these signals, cells activate intracellular signaling pathways that lead to the production and release of interferons. The released interferons then bind to specific receptors on neighboring cells, initiating a cascade of cellular responses.
The Main Functions of Interferons
- Antiviral Defense: Interferons play a crucial role in limiting viral replication and spread. They induce an antiviral state in nearby cells by upregulating the expression of various antiviral proteins, such as protein kinase R (PKR), 2'-5'-oligoadenylate synthetase (OAS), and Mx proteins. These proteins inhibit viral replication and enhance the immune response against viruses.
- Activation of Immune Cells: Interferons activate and modulate the functions of immune cells, including natural killer (NK) cells, macrophages, and dendritic cells. They enhance the cytotoxic activity of NK cells and help macrophages and dendritic cells to efficiently present antigens, thereby promoting an effective immune response against infected cells.
- Regulation of Adaptive Immunity: Interferons influence the adaptive immune response by regulating the differentiation and activation of T and B lymphocytes. They promote the development of a Th1 immune response, which is characterized by the production of IFN-gamma and the activation of cytotoxic T cells. IFN-gamma also enhances the antibody production by B cells.
- Immunomodulatory Effects: Interferons have immunomodulatory effects, influencing the balance between pro-inflammatory and anti-inflammatory responses. They can promote inflammation to eliminate pathogens but also have anti-inflammatory functions to prevent excessive immune activation and tissue damage.
- Antitumor Activity: Interferons have been used in cancer therapy due to their ability to inhibit tumor cell growth and induce apoptosis. They can modulate the tumor microenvironment, enhance the immune recognition of tumor cells, and activate immune-mediated tumor cell killing.
Interferons have been extensively studied and utilized in medicine, both as therapeutic agents and as diagnostic tools. Recombinant interferons have been developed and used for the treatment of viral infections (such as hepatitis B and C), certain cancers (such as melanoma and leukemia), and immune-related disorders (such as multiple sclerosis). They have also been investigated for potential applications in antiviral vaccines and immunotherapies.
Mechanism of Action of Interferons
The mechanism of action of interferons (IFNs) involves a complex cascade of cellular and molecular events that collectively contribute to their antiviral, immunomodulatory, and antitumor effects. The following steps outline the general mechanism of action of IFNs:
- Induction of Interferon Production: Interferon production is induced in response to viral infections or other immune stimuli. The recognition of viral RNA or other pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) triggers intracellular signaling pathways, leading to the activation of transcription factors, such as interferon regulatory factors (IRFs) and nuclear factor-kappa B (NF-κB). These transcription factors promote the expression of interferon genes.
- Interferon Secretion: Once induced, interferons are synthesized and released by infected cells or other immune cells. The main types of interferons include Type I (IFN-alpha and IFN-beta), Type II (IFN-gamma), and Type III (IFN-lambda). They are secreted into the extracellular space.
- Binding to Interferon Receptors: Interferons bind to specific cell surface receptors on neighboring cells. The receptors differ depending on the type of interferon. Type I interferons bind to the IFNAR receptor complex, which consists of IFNAR1 and IFNAR2 subunits. Type II interferon (IFN-gamma) binds to the IFNGR receptor, composed of IFNGR1 and IFNGR2 subunits. Type III interferons (IFN-lambda) bind to the IL-10 receptor subunit IL-10R2 and the IFN-lambda-specific receptor subunit IL-28Rα or IL-10R2 and IL-10R1.
- Activation of JAK-STAT Signaling Pathway: Interferon binding to its receptor triggers the activation of Janus kinases (JAKs), which are associated with the intracellular domain of the receptor. JAKs phosphorylate and activate signal transducer and activator of transcription (STAT) proteins, including STAT1 and STAT2.
- Formation of Interferon-Stimulated Gene Factor 3 (ISGF3) Complex: Phosphorylated STAT1 and STAT2, along with IRF9, form a heterotrimeric complex called interferon-stimulated gene factor 3 (ISGF3). ISGF3 translocates to the nucleus, where it binds to specific interferon-stimulated response elements (ISREs) in the promoter regions of interferon-stimulated genes (ISGs).
- Induction of Interferon-Stimulated Genes (ISGs): ISGF3 binding to ISREs leads to the transcriptional activation of numerous ISGs. These ISGs encode proteins with antiviral, immunomodulatory, and antiproliferative functions. Examples of ISGs include protein kinase R (PKR), 2'-5'-oligoadenylate synthetase (OAS), myxovirus resistance proteins (Mx proteins), and major histocompatibility complex (MHC) molecules.
- Antiviral Response: The induction of ISGs by interferons results in the production of antiviral proteins that inhibit viral replication and spread. For example, PKR phosphorylates and inactivates translation initiation factor eIF2, leading to the inhibition of viral protein synthesis. OAS produces 2'-5'-oligoadenylates, which activate RNase L, leading to degradation of viral RNA. Mx proteins interfere with viral replication cycles.
- Immunomodulation and Antitumor Effects: Interferons also exert immunomodulatory effects by enhancing the activity of immune cells, such as natural killer (NK) cells, macrophages, and dendritic cells. They promote the cytotoxicity of NK cells, enhance antigen presentation, and modulate the balance between pro-inflammatory and anti-inflammatory responses. Additionally, interferons have antitumor effects by inhibiting tumor cell proliferation, inducing apoptosis, and modulating the tumor microenvironment.
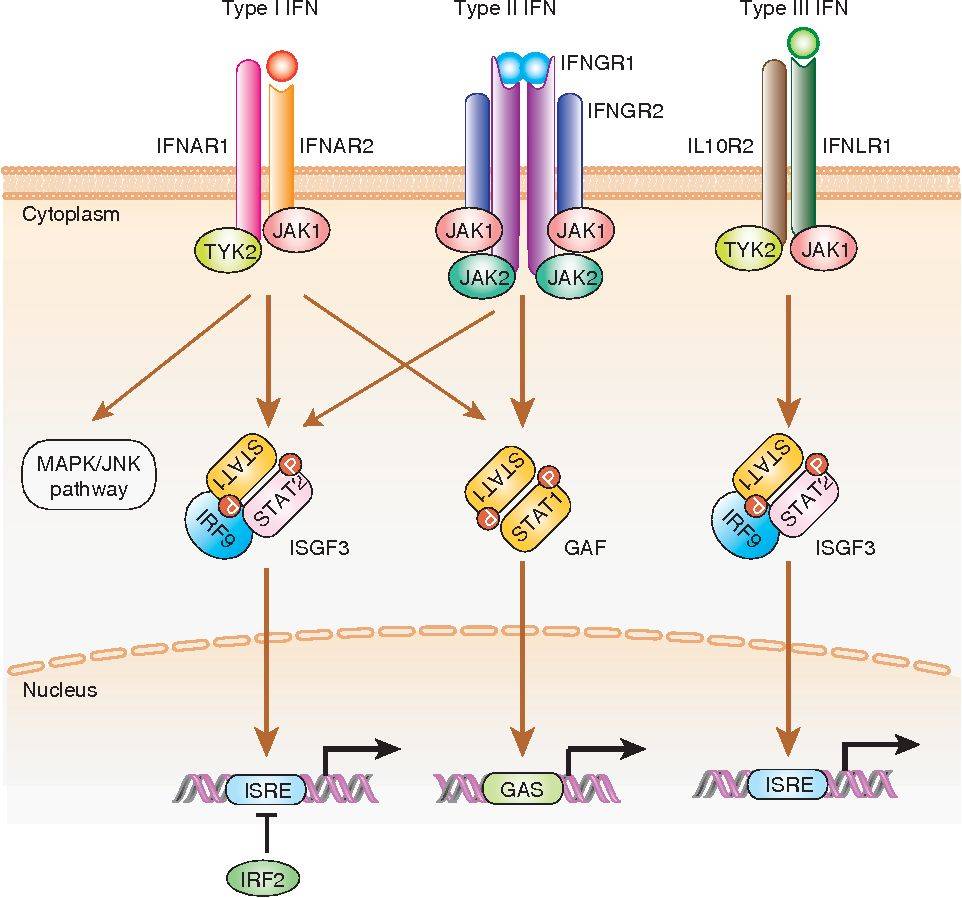 Fig.2 Signal transduction by type I, type II, and type III interferon (IFN) receptors. (Negishi H, 2018)
Fig.2 Signal transduction by type I, type II, and type III interferon (IFN) receptors. (Negishi H, 2018)
It's important to note that the specific mechanisms may vary depending on the type of interferon and the target cell type. The overall impact of interferons is the activation of antiviral defenses, modulation of immune responses, and inhibition of cell proliferation, collectively contributing to the control of viral infections, immunomodulation, and antitumor effects.
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Khanna NR, Gerriets V. Interferon. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 10, 2023.
- Negishi H, Taniguchi T, Yanai H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb Perspect Biol. 2018;10(11):a028423.

