What is TTR Protein
Transthyretin (TTR), also known by its official full name "prealbumin," is a versatile protein that plays a pivotal role in human physiology. This protein, belonging to the albuminoid superfamily, serves various crucial functions within the body. Synonyms for TTR include thyroxine-binding prealbumin and TTR-related amyloidosis.
TTR Protein Structural Characteristics and Classification
Structurally, TTR is a tetrameric protein composed of four identical subunits, each exhibiting a distinctive beta-sheet structure. The classification of TTR places it in the family of carrier proteins, given its role in transporting thyroid hormones and vitamin A. Recent research advances in TTR have shed light on its intricate structure-function relationship, providing deeper insights into its physiological significance.
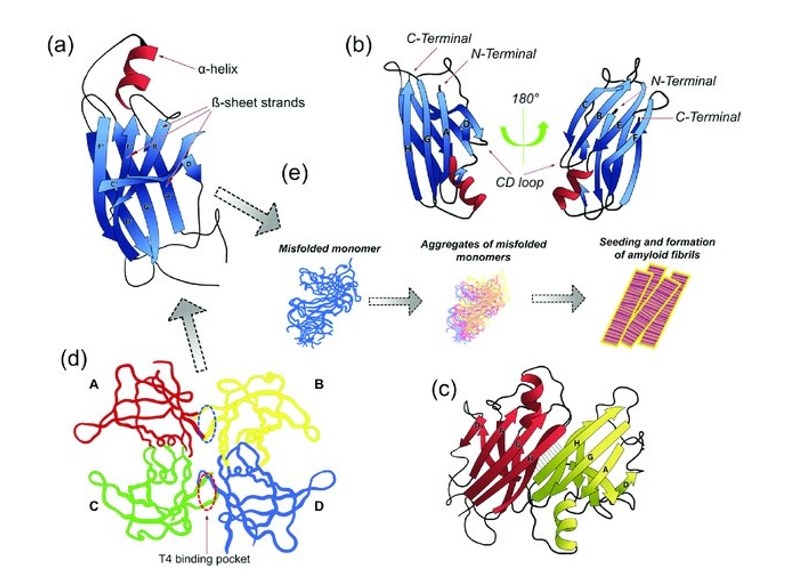
Figure 1. Transthyretin (TTR) conformational structures. (Gonzalez-Duarte A, et al., 2021)
TTR Biological Functions and Molecular Mechanisms
The biological functions of TTR are multi-faceted, underscoring its indispensability in maintaining homeostasis. Principally, TTR acts as a carrier protein for thyroxine (T4) and retinol-binding protein, facilitating the transport of these vital molecules in the bloodstream. Additionally, TTR has been implicated in the regulation of beta-amyloid peptide levels, suggesting a potential link between TTR and neurodegenerative diseases.
At the molecular level, TTR's function involves the binding and transportation of its ligands. The tetrameric structure of TTR enables efficient binding and release of thyroid hormones and vitamin A, ensuring their proper distribution and utilization in various tissues. The intricate molecular mechanisms governing these processes continue to be a subject of active research.
TTR Related Signaling Pathway
The signaling pathways associated with TTR are intricate and intertwined with various physiological processes. TTR's involvement in thyroid hormone transport implicates it in the regulation of metabolic pathways and energy homeostasis. Moreover, the interplay between TTR and beta-amyloid peptides suggests a potential role in neurodegenerative pathways.
Elucidating the signal pathways modulated by TTR is imperative for deciphering its broader physiological impact. Researchers are delving into the intricate network of interactions involving TTR to delineate its role in health and disease, providing a foundation for targeted therapeutic interventions.
TTR Related Diseases
While TTR is an indispensable player in normal physiology, its dysregulation is associated with several pathological conditions. TTR-related amyloidosis (ATTR) stands out as a prominent disease linked to this protein. In ATTR, misfolded TTR aggregates form amyloid deposits in various tissues, compromising organ function. The disease manifests in different forms, including familial amyloidotic polyneuropathy (FAP) and senile systemic amyloidosis (SSA), each presenting unique clinical challenges.
Understanding the etiology of TTR-related diseases is crucial for developing targeted therapeutic interventions. Research efforts have been directed towards unraveling the factors contributing to TTR misfolding and aggregation, providing a foundation for the development of novel treatment strategies.
TTR's Applications in Biomedicine
Beyond its physiological significance, TTR has garnered attention for its applications in biomedical research and development. The protein's unique properties make it a valuable asset in diagnostic development, vaccine research, and therapeutic strategies.
In diagnostics, TTR serves as a biomarker for assessing thyroid and nutritional status. Researchers have harnessed its binding affinity for thyroxine to develop diagnostic assays, enabling precise evaluation of thyroid function.
The potential role of TTR in vaccine development stems from its ability to transport retinol, a crucial component for immune function. Exploring TTR as a carrier for vaccine antigens holds promise in enhancing vaccine efficacy and promoting targeted immune responses.
In therapeutics, TTR has emerged as a potential target for addressing amyloid-related diseases. Ongoing research endeavors are focused on developing small molecules and biologics that can modulate TTR stability and prevent pathological aggregation, offering hope for effective treatments for TTR-related amyloidosis.
Recommended Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| TTR-3950H | Recombinant Human TTR protein, His-tagged | Human | HEK293 | His |
| TTR-31111TH | Recombinant Human TTR | Human | N/A | |
| TTR-11H | Recombinant Human TTR | Human | Trichoplusia Ni Larval | N/A |
| TTR-728H | Recombinant Human TTR protein, His-tagged | Human | E.coli | His |
| TTR-2774H | Recombinant Human TTR Protein, His-tagged | Human | E.coli | N-His |
| TTR-3880H | Recombinant Human TTR protein, His-tagged | Human | HEK293 | His |
| TTR-1220HFL | Recombinant Full Length Human TTR Protein, C-Flag-tagged | Human | Mammalian cells | Flag |
| TTR-6215H | Recombinant Human TTR Protein, Myc/DDK-tagged, C13 and N15-labeled | Human | HEK293T | Myc/DDK |
| TTR-128H | Recombinant Human TTR Protein, His-tagged | Human | E.coli | His |
| TTR-2922H | Recombinant Human TTR Protein, MYC/DDK-tagged | Human | HEK293 | Myc/DDK |
Reference
- Gonzalez-Duarte A, Ulloa-Aguirre A. A brief journey through protein misfolding in transthyretin amyloidosis (ATTR amyloidosis). International Journal of Molecular Sciences. 2021, 22(23): 13158.

