MBP
-
Official Full Name
myelin basic protein -
Overview
The protein encoded by the classic Mbp gene is a major constituent of the myelin sheath of oligodendrocytes and Schwann cells in the nervous system. However, Mbp-related transcripts are also present in the bone marrow and the immune system. These mRNAs arise from the long Mbp gene (otherwise called "Golli-Mbp") that contains 3 additional exons located upstream of the classic Mbp exons. Alternative splicing from the Golli and the Mbp transcription start sites gives rise to 2 sets of Mbp-related transcripts and gene products. The Golli mRNAs contain 3 exons unique to Golli-Mbp, spliced in-frame to 1 or more Mbp exons. They encode hybrid proteins that have N-terminal Golli aa sequence linked to Mbp aa sequence. The second family of transcripts contain only Mbp exons and produce the well characterized myelin basic proteins. This complex gene structure is conserved among species suggesting that the Mbp transcription unit is an integral part of the Golli transcription unit and that this arrangement is important for the function and/or regulation of these genes. Mutation of the Mbp gene is associated with the shiverer and myelin deficient phenotypes in mouse. [provided by RefSeq, Jul 2008] -
Synonyms
MBP;myelin basic protein;mld;shi;Hmbpr;C76307;R75289;golli-mbp;shiverer;myelin deficient;myelin A1 protein
Recombinant Proteins
- Human
- Pig
- Mouse
- E.coli
- Chicken
- Bovine
- Rabbit
- Cattle
- Pan-species
- E.coli
- Mouse Central Nervous System
- Mammalian Cells
- HEK293
- Human Brain
- Porcine Brain
- Bovine Central Nervous System Tissue
- Yeast
- Swine
- Pig Brain
- GST
- Non
- His
- T7
- DDK
- Myc
- SUMO
- Flag
- Avi
- Fc
- GFP
Background
What is MBP protein?
MBP gene (myelin basic protein) is a protein coding gene which situated on the long arm of chromosome 18 at locus 18q23. The protein encoded by the classic MBP gene is a major constituent of the myelin sheath of oligodendrocytes and Schwann cells in the nervous system. However, MBP-related transcripts are also present in the bone marrow and the immune system. MBP is the second most abundant protein in the myelin sheath of the central nervous system and is mainly responsible for maintaining the adhesion of the cytoplasmic surface of the multiple layers of tight myelin sheath. MBP is also a 42kDa protein that exists in the periplasmic space of bacteria and is involved in the transport of maltose and maltodextrin across bacterial cell membranes. The MBP protein is consisted of 304 amino acids and MBP molecular weight is approximately 33.1 kDa.
What is the function of MBP protein?
MBP belongs to a family of "intrinsically disordered" or conformationally adaptable proteins that have a variety of other functions in addition to their role in myelination. For example, it can interact with a variety of polyanionic proteins (such as actin, tubulin, Ca^2+^ -calmodulin, and clathrin) as well as negatively charged lipids, and gain structure when it binds to them. In addition, some large and small subtypes of MBP can be transported into the nucleus, so they may also bind to polynucleotides. It may act as a membrane actin-binding protein, potentially participating in the transmission of extracellular signals to the cytoskeleton in oligodendrocytes and tight junctions in myelin. In addition to its structural role, MBP has been implicated in various biological processes, including host defense against parasitic infections, activation of immune cells like mast cells, basophils, and neutrophils, complement activation, platelet agonist activity, antibacterial activity, and acting as a natural protein inhibitor of heparinase.
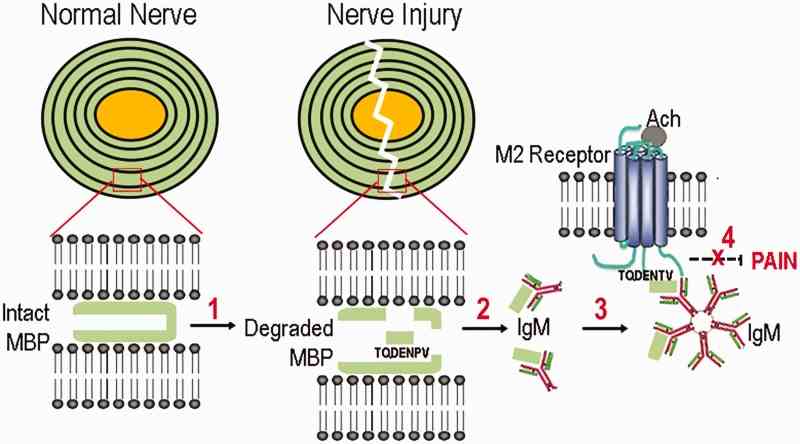
Fig1. MBP/acetylcholine receptor homology model of pain (a hypothesis diagram). (Veronica I Shubayev, 2018)
MBP Related Signaling Pathway
In terms of signal transduction, MBP itself is not directly involved in the signal transduction pathway, but it is associated with a variety of signal pathways. For example, MBP is closely associated with oligodendrocyte maturation and myelination, and these processes are regulated by multiple cell signaling pathways. In addition, the expression and function of MBP may be influenced by signaling pathways such as TGF-β/ activin and BMP, which signal through members of the Smad protein family to influence cell proliferation, differentiation, and cell fate determination.
BMP signaling relies on heterotetramer serine/threonine kinase receptors, including BMPRI (BMP receptor type I) and BMPRII (BMP receptor type II). BMP binding to its receptor complex leads to activation of BMPRI, which phosphorylates Smad1, Smad5, and Smad8 molecules. These phosphorylated Smad molecules then form complexes with co-SMAD, or Smad4, that migrate into the nucleus to activate transcription of specific genes.
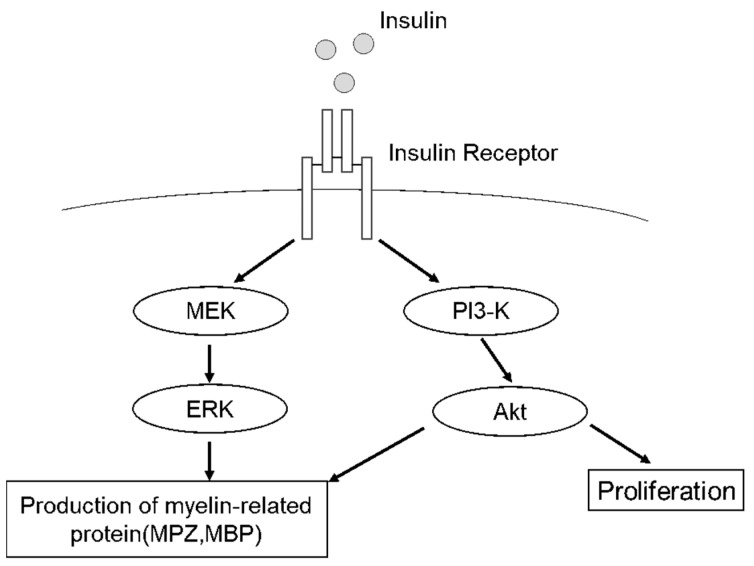
Fig1. Proposed scheme for insulin-stimulated signaling of the proliferation and the production of myelin-related proteins. (Tomokazu Saiki, 2021)
MBP Related Diseases
Because MBP is a key component of the myelin sheath of the central nervous system, abnormalities in it are often associated with diseases that affect nerve conduction. MBP is a major component of the myelin sheath, and its destruction leads to the obstruction of nerve signaling, resulting in Multiple Sclerosis (MS). Other demyelinating diseases include, but are not limited to, inherited metabolic disorders such as adrenoleukodystrophy and Krabbe disease, which can also cause damage to the myelin sheath. The abnormality of MBP may affect the repair process after nerve injury. In addition, meningitis or encephalitis caused by certain viruses and bacteria may cause neurological impairment by affecting the structure of the MBP and myelin sheath.
Bioapplications of MBP
MBP is also widely used as a fusion tag in protein expression and purification. It not only increases the solubility of the target protein, but also provides one-step purification through affinity with the cross-linked starch resin. In addition, when expressed in mammalian cells, the fusion protein of the MBP label is generally able to enhance protein production and reduce the number of cell deaths. Studies have shown that MBP fusion proteins, when expressed in eukaryotic cells, usually exist as monomers and do not cause N-glycosylation, but may cause O-glycosylation. In terms of disease treatment, MBP can be used as a biomarker for monitoring central nervous system damage and demyelinating diseases, and changes in its levels in the cerebrospinal fluid or blood may indicate disease activity and severity. The structural and functional properties of MBP make it a target for drug screening, especially in the search for drugs that can promote myelin regeneration or inhibit inflammation.
Case Study
Case Study 1: Jocelyn G Labombarde, 2022
Infection and vaccination repeatedly expose individuals to antigens that are conserved between influenza virus subtypes. Nevertheless, antibodies recognizing variable influenza epitopes greatly outnumber antibodies reactive against conserved epitopes. Elucidating factors contributing to the paucity of broadly reactive influenza antibodies remains a major obstacle for developing a universal influenza vaccine. Here, the experimental autoimmune encephalitis (EAE) of mice was induced by injecting mouse native myelin basic protein. Thus researchers report that inducing broadly reactive influenza antibodies increases autoreactive antibodies in humans and mice and exacerbates disease in four distinct models of autoimmune disease. Importantly, transferring broadly reactive influenza antibodies augments disease in the presence of inflammation or autoimmune susceptibility. Further, broadly reactive influenza antibodies spontaneously arise in mice with defects in B cell tolerance.
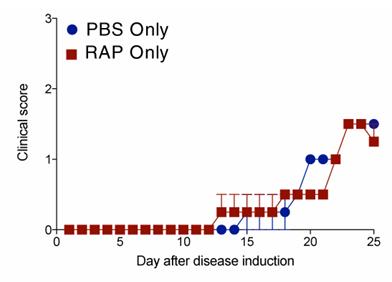
Fig1. Mice were treated daily with rapamycin or PBS, immunized with native MBP in CFA, and scored daily for disease.
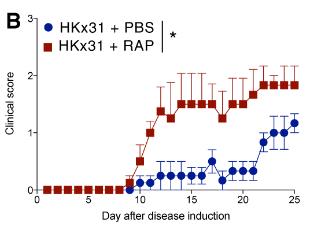
Fig2. Mice were treated daily with HKx31and rapamycin or PBS and scored daily for disease.
Case Study 2: Nicholas Johnson, 2012
Whilst the co-translational translocation of nascent proteins across the mammalian endoplasmic reticulum (ER) is well defined, the capacity of this organelle for post-translational translocation is poorly delineated. Here researchers identify two human secretory protein precursors, apelin and statherin, as bona fide substrates for post-translational translocation across the ER membrane. MBP protein was used in WRBcc inhibition assay. Further studies, in combination with Hyalophora cecropia preprocecropin A (ppcecA), show that all three proteins bind to TRC40 and can utilise this component for their delivery to the ER membrane in a well-established in vitro system. However, ppcecA is not an obligate TRC40 substrate, and it can also be delivered to the ER by an alternative TRC40-independent pathway. Upon arrival at the ER membrane, these short secretory proteins appear to be ubiquitously transported across the ER membrane through the Sec61 translocon, apparently irrespective of their delivery route.
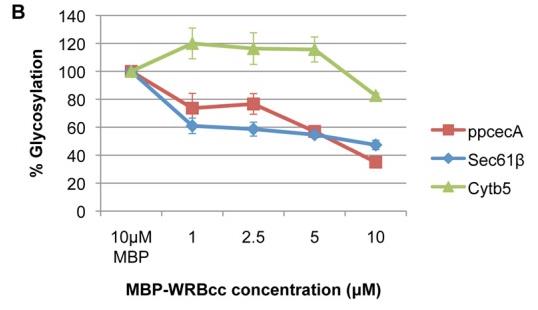
Fig3. Graphical representation of relative N-glycosylation of proteins in response to increasing concentrations of MBP-WRBcc.
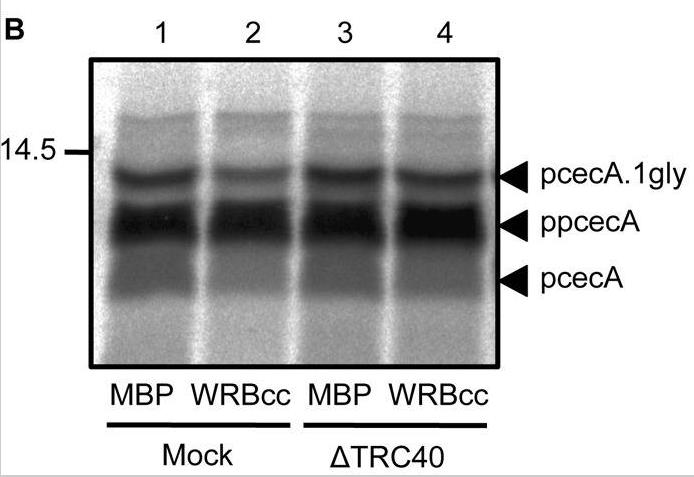
Fig4. Rabbit reticulocyte lysate was immunodepleted for TRC40 or subjected to a mock treatment.
Quality Guarantee
High Purity
.jpg)
Fig1. SDS-PAGE (MBP-476H)
.
.jpg)
Fig2. SDS-PAGE (MBP-2763H)
Involved Pathway
MBP involved in several pathways and played different roles in them. We selected most pathways MBP participated on our site, such as Glial Cell Differentiation,MAPK Cascade,Neural Crest Differentiation, which may be useful for your reference. Also, other proteins which involved in the same pathway with MBP were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Spinal Cord Injury | GDNF,GAP43,AIF1,NOX4,KLK8,RTN4R,RGMA,LILRB3,LGALS3,PTPRZ1 |
| Neural Crest Differentiation | TXLNB,MYB,ASCL1,DMBX1,CDH6,OLIG1,GBX2,DCT,DLX5,HOXA1 |
| Glial Cell Differentiation | PLP1,GAP43,CNP,TPPP |
| MAPK Cascade | MAP2,MAP3K2,MBPB |
Protein Function
MBP has several biochemical functions, for example, protease binding,protein binding,structural constituent of myelin sheath. Some of the functions are cooperated with other proteins, some of the functions could acted by MBP itself. We selected most functions MBP had, and list some proteins which have the same functions with MBP. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| protease binding | CD70,FN1,SERPINA1,RNF139,ITGAV,BCL2,POLG,RFFL,DPP4,ALPI |
| protein binding | CEP290,RWDD2B,HSPB2,KCNRG,LGALSL,LGALS2,TERF2IP,FCGR2A,NDUFB10,PID1 |
| structural constituent of myelin sheath | NCMAP,MBPB,MOBP,MAL,PLP1 |
Interacting Protein
MBP has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with MBP here. Most of them are supplied by our site. Hope this information will be useful for your research of MBP.
CTDSP1;q8iin1_plaf7;PBK;PRMT10;HLA-DRB1;RXRB;ATXN1;ATN1;PDGFRL;APTX;APP;HLA-DRB1;HLA-DMA
Resources
Related Services
Related Products
References
- Rao, QC; Hsieh, YHP; et al. Effect of pH, temperature and storage time on the stability of bovine myelin basic protein. FOOD CONTROL 50:166-172(2015).
- Saffari, H; Kennedy, A; et al. NON-INVASIVE ULTRASOUND TOIDENTIFY EOSINOPHIL GRANULE PROTEINS IN EOSINOPHILIC ESOPHAGITIS. ULTRASOUND IN MEDICINE AND BIOLOGY 41:884-889(2015).





