Bovine Myelin basic protein
| Cat.No. : | MBP-6950B |
| Product Overview : | Bovine Myelinbasic protein, was highly Purified from bovinecentral nervous system tissue by method of Määttä et al. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Bovine |
| Source : | Bovine Central Nervous System Tissue |
| Tag : | Non |
| Description : | MBP is a peripheralmembrane protein, and its interaction with lipids is generally believed to becritical for the formation and stability of the multilamellar myelin sheath.It has been identified as a Ca2+-calmodulin regulated agent foractin polymerization, tropomyosin and actomyosin function, tubulinstabilization, and clathrin assembley. MBP is generally considered to be theantigen responsible for autoimmunity in multiple sclerosis (MS) and it hasthe capability to induce experimental allergic encephalomyelitis (EAE). MBPis also an endogenous inhibitor of the high-affinity cannabinoid binding sitein the brain and a substrate for numerous protein kinases (MAPK,serine/threonine kinase, protein kinase C). MBP is also suggested to beinvolved in intracellular signalling and as cytoskeletal components. |
| Purity : | ≥95% (SDS-PAGE; immunoblot) |
| Formulation : | Lyophilized. Essentially salt-free. |
| Reconstitution : | Reconstitute with distilled water. |
| Application : | Substratefor various kinases including MAPK, PKA, calmodulin-dependent protein kinase,PKC and phosphorylase kinase. Raf1, MEK, and MEKK can also use MBP as asubstrate. |
| Storage : | Stablefor at least 1 year when stored at +4°C. Reconstituted protein is stable forat least 6 months at -20°C and for at least 24 hours at +37°C in aqueoussolution. |
| Gene Name | MBP myelin basic protein [ Bostaurus ] |
| Official Symbol | MBP |
| Synonyms | MBP;myelin basic protein; myelin A1 protein; microtubule-stabilizing protein; 20kDa microtubule-stabilizing protein |
| Gene ID | 618684 |
| mRNA Refseq | NM_001206674 |
| Protein Refseq | NP_001193603 |
| Chromosome Location | 24q11-q13.2 |
| Function | structuralconstituent of myelin sheath |
| ◆ Recombinant Proteins | ||
| MBP-6832C | Recombinant Chicken MBP | +Inquiry |
| MBP-50P | Recombinant Pig MBP protein, His-tagged | +Inquiry |
| MBP-2348H | Recombinant Human MBP, His-tagged | +Inquiry |
| MBP-1771R | Recombinant Rabbit MBP protein, His & GST-tagged | +Inquiry |
| MBP-2527H | Recombinant Human MBP protein(101-190 aa), C-His-tagged | +Inquiry |
| ◆ Native Proteins | ||
| MBP-99S | Native Swine MBP | +Inquiry |
| MBP-6949M | Native Mouse Myelin basic protein | +Inquiry |
| MBP-89S | Native Swine MBP Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| MBP-4437HCL | Recombinant Human MBP 293 Cell Lysate | +Inquiry |
| MBP-4436HCL | Recombinant Human MBP 293 Cell Lysate | +Inquiry |
| MBP-4438HCL | Recombinant Human MBP 293 Cell Lysate | +Inquiry |
TRC40 can deliver short secretory proteins to the Sec61 translocon
Journal: Journal of Cell Science PubMed ID: 22505607 Data: 2012/8/1
Authors: Nicholas Johnson, Fabio Vilardi, Stephen High
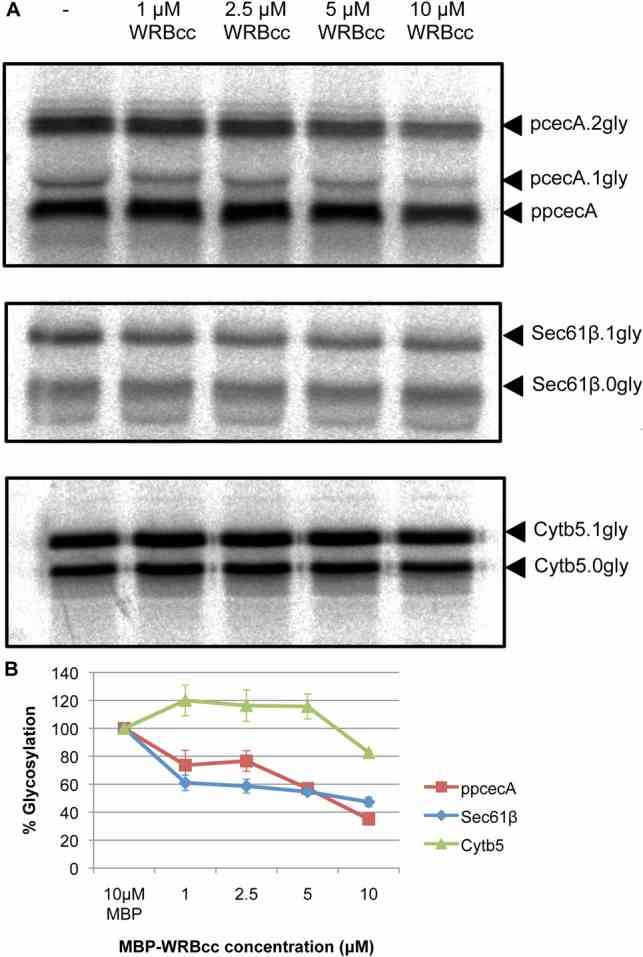
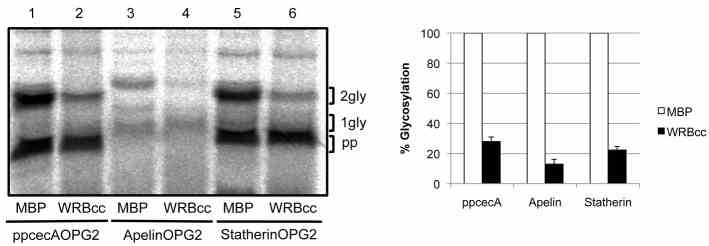
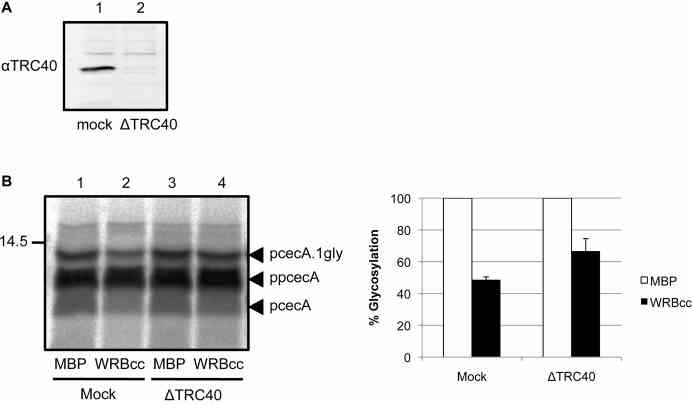
Article Snippet:Proteins were synthesised using nuclease treated or untreated rabbit reticulocyte lysate in the presence of [ 35 S]methionine for 15 minutes at 30°C as before.Proteins were synthesised using nuclease treated or untreated rabbit reticulocyte lysate in the presence of [ 35 S]methionine for 15 minutes at 30°C as before.. The reaction was treated with 1 mM puromycin, immediately split, and MBP (Creative BioMart) or MBP-WRBcc ( ) added to 10 μM.. The reaction was incubated for 10 minutes at 30°C prior to addition of microsomes.The reaction was incubated for 10 minutes at 30°C prior to addition of microsomes.
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All MBP Products
Required fields are marked with *
My Review for All MBP Products
Required fields are marked with *



