Recombinant Human STAT3, GST-tagged
| Cat.No. : | STAT3-1496H |
| Product Overview : | Recombinant full-length human STAT3 was expressed by baculovirus inSf9insect cells using an N-terminal GST tag. MW = 120 kDa. |
| Availability | January 25, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Sf9 Cells |
| Tag : | GST |
| Description : | STAT3 is a member of the signal transducers and activators of transcription (STAT) family of proteins that carry out a dual function: signal transduction and activation of transcription. STAT3 is widely expressed and becomes activated through phosphorylation on tyrosine as a DNA binding protein in response to a variety of stimuli such as EGF, IL-6, PDGF, IL-2 and G-CSF. This phosphoprotein forms homodimers as well as heterodimers with other members of the STAT family and translocate to the nucleus in order to modulate the transcription of various genes. |
| Sequence : | Full-length. |
| Applications : | Kinase Assay, Western Blot. |
| Storage And Stability : | Store product at -70℃. For optimal storage, aliquot target into smaller quantities after centrifugation and store at recommended temperature. For most favorable performance, avoid repeated handling and multiple freeze/thaw cycles. |
| Publications : |
|
| Gene Name | STAT3 signal transducer and activator of transcription 3 (acute-phase response factor) [ Homo sapiens ] |
| Synonyms | STAT3; signal transducer and activator of transcription 3 (acute-phase response factor); APRF; HIES; FLJ20882; MGC16063; signal transducer and activator of transcription 3; DNA-binding protein APRF; acute-phase response factor |
| Gene ID | 6774 |
| mRNA Refseq | NM_003150 |
| Protein Refseq | NP_003141 |
| MIM | 102582 |
| UniProt ID | P40763 |
| Chromosome Location | 17q21.31 |
| Pathway | Acute myeloid leukemia; Adipocytokine signaling pathway; Chemokine signaling pathway; Jak-STAT signaling pathway; Pancreatic cancer; Pathways in cancer; Signaling by PDGF; Signalling by NGF |
| Function | calcium ion binding; hematopoietin/interferon-class (D200-domain) cytokine receptor signal transducer activity; protein dimerization activity; protein kinase binding; sequence-specific DNA binding; signal transducer activity; transcription activator activity; transcription factor activity; transcription factor binding |
| ◆ Recombinant Proteins | ||
| STAT3-2176H | Recombinant Human STAT3, His-tagged | +Inquiry |
| STAT3-4148H | Recombinant Human STAT3 Protein, His (Fc)-Avi-tagged | +Inquiry |
| STAT3-2174H | Recombinant Human STAT3, MYC/DDK-tagged | +Inquiry |
| STAT3-335H | Recombinant Human STAT3 protein, His/MBP-tagged | +Inquiry |
| STAT3-2837H | Recombinant Human STAT3 Protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| STAT3-1417HCL | Recombinant Human STAT3 293 Cell Lysate | +Inquiry |
| STAT3-1418HCL | Recombinant Human STAT3 293 Cell Lysate | +Inquiry |
| STAT3-301HKCL | Human STAT3 Knockdown Cell Lysate | +Inquiry |
Suppression of Breast Cancer Cell Proliferation by Selective Single-Domain Antibody for Intracellular STAT3
Journal: Breast Cancer : Basic and Clinical Research PubMed ID: 29434474 Data: 2018/1/3
Authors: Sunanda Singh, Genoveva Murillo, Rajendra G Mehta
Article Snippet:Recombinant full-length human STAT3 with a GST tag fused to its N-terminal (STAT3-1496H) was provided by Creative BioMart (Shirley, NY, USA).. VHH discovery was achieved.VHH discovery was achieved.
Blockade of Glioma Proliferation Through Allosteric Inhibition of JAK2
Journal: Science signaling PubMed ID: 23838182 Data: 2016/3/1
Authors: Kunyan He, Qi Qi, Keqiang Ye
Article Snippet:All antibodies and the GSH depletion kit were from Cell Signaling Technology.All antibodies and the GSH depletion kit were from Cell Signaling Technology.. Glutathione S-transferase (GST)–STAT3 protein was purchased from Creative BioMart.. Nude mice (nu/nu), 5 to 6 weeks of age, were obtained from the National Cancer Institute (NCI).Nude mice (nu/nu), 5 to 6 weeks of age, were obtained from the National Cancer Institute (NCI).
mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
Journal: PLoS Genetics PubMed ID: 26241748 Data: 2015/8/4
Authors: Bin Huang, Yongkui Wang, Gregory S. Barsh
Article Snippet:Beads were then washed four times with lysis buffer and once kinase buffer (25mM HEPES (pH7.4), 50mM KCl, 10mM MgCl2, 250 μM ATP).Beads were then washed four times with lysis buffer and once kinase buffer (25mM HEPES (pH7.4), 50mM KCl, 10mM MgCl2, 250 μM ATP).. 0.4μg of recombinant GST-tagged full-length STAT3 peptide (Creative BioMart, #1496H) was added to 30μl kinase buffer.. Kinase assays were performed for 30 min at 30°C, and terminated by the addition of the 2×SDS sample buffer followed by boiling for 5 min.Kinase assays were performed for 30 min at 30°C, and terminated by the addition of the 2×SDS sample buffer followed by boiling for 5 min.
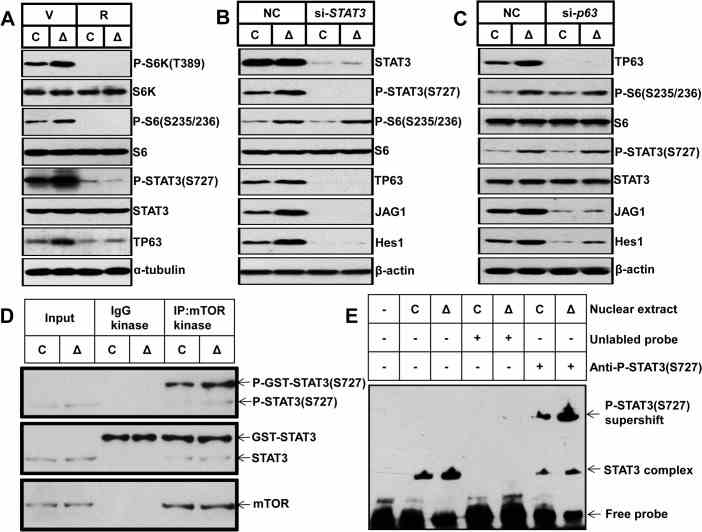
(A) Control and Δ Tsc1 primary calvarial cells were treated with vehicle (V) or 0.1 nM rapamycin (R) for 24 hours and then underwent immunoblotting to detect phosphorylation of STAT3 (Ser727) and TP63 (total p63). Control and Δ Tsc1 primary calvarial cells were treated with STAT3 (B) and p63 (C) siRNA and negative control (NC) for 48 hours and then underwent immunoblotting to detect mTORC1 activity, phosphorylation of STAT3(Ser727), TP63 (total p63) and activity of Notch pathway (JAG1, Hes1). (D) Cultured primary calvarial cells were immunoprecipitated with anti-mTOR antibody and the precipitated mTOR was assayed for kinase activity
Case 1: Huang B, et al. PLoS Genet. 2015
The mechanistic target of rapamycin (mTOR) integrates both intracellular and extracellular signals to regulate cell growth and metabolism. However, the role of mTOR signaling in osteoblast differentiation and bone formation is undefined, and the underlying mechanisms have not been elucidated.
Here, the researchers report that activation of mTOR complex 1 (mTORC1) is required for preosteoblast proliferation; however, inactivation of mTORC1 is essential for their differentiation and maturation. Inhibition of mTORC1 prevented preosteoblast proliferation, but enhanced their differentiation in vitro and in mice. In vitro, rhSTAT3 was added into the buffer to detect kinase assay for mTORC1. The results showed, mTORC1 prevented osteoblast maturation through activation of the STAT3/p63/Jagged/Notch pathway and downregulation of Runx2. And the role of mTORC1 in osteoblast formation and established that mTORC1 prevents preosteoblast differentiation and maturation through activation of the Notch pathway.
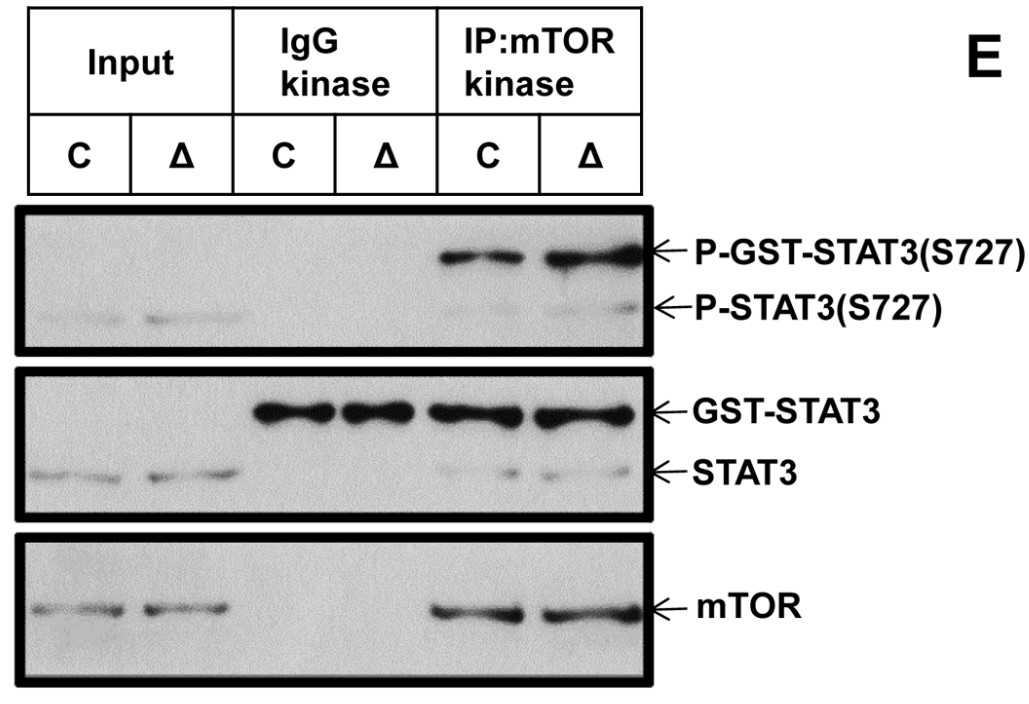
Fig1. The kinase assay for mTORC1 against recombinant GST-tagged full-length STAT3.
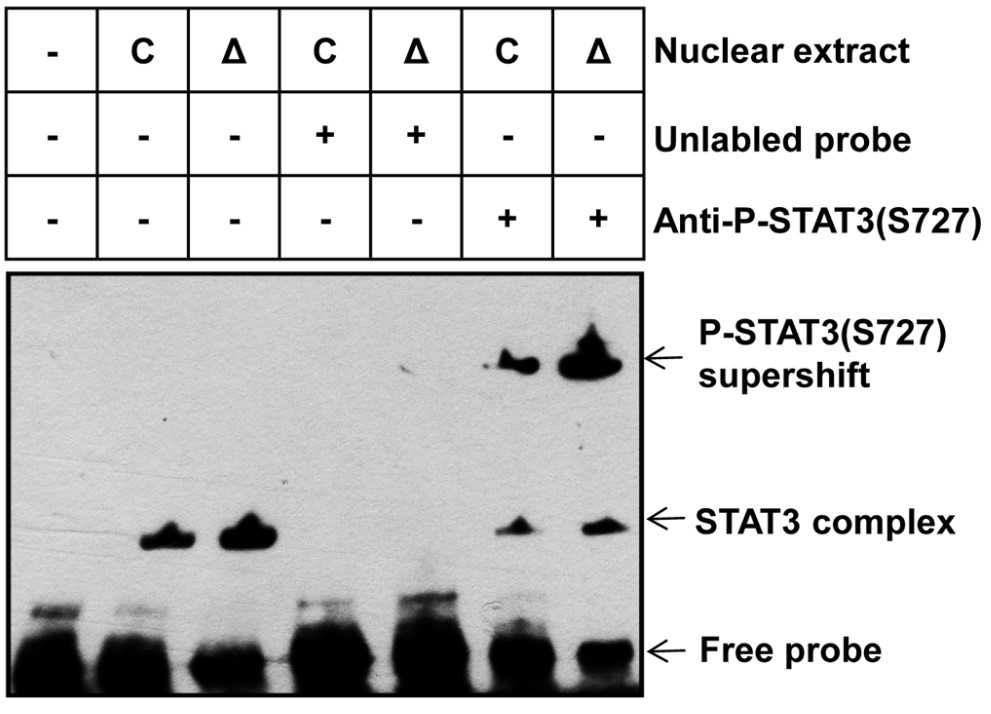
Fig2. Nuclear extract from control and ΔTsc1 primary calvarial cells were analyzed using EMSA.
Case 2: He K, et al. Sci Signal. 2013
The gene that encodes the epidermal growth factor receptor (EGFR) is frequently overexpressed or mutated in human cancers, including glioblastoma. However, the efficacy of EGFR-targeted small-molecule inhibitors or monoclonal antibodies in glioblastomas that also have mutation or deletion of the gene encoding phosphatase and tensin homolog (PTEN) has been modest. The researchers found that EGFR signaling was blocked by a small molecule (G5-7) that selectively inhibited Janus kinase 2 (JAK2)-mediated phosphorylation and activation of EGFR and STAT3 (signal transducer and activator of transcription 3) by binding to JAK2, thereby decreasing the activity of downstream signaling by mTOR (mammalian target of rapamycin) and inducing cell cycle arrest.
The effect of G5-7 on kinase activity was tested and the rhSTAT3 was used as a substract for JAK2 kinase assay. G5-7 reduced vascular endothelial growth factor (VEGF) secretion and endothelial cell migration and induced apoptosis in glioblastoma xenografts, thereby suppressing glioblastoma growth in vivo. Furthermore, G5-7 was more potent than EGFR or JAK2 inhibitors that interfere with either ligand or adenosine 5'-triphosphate (ATP) binding at impeding glioblastoma cell proliferation, demonstrating that this allosteric JAK2 inhibitor may be an effective clinical strategy.
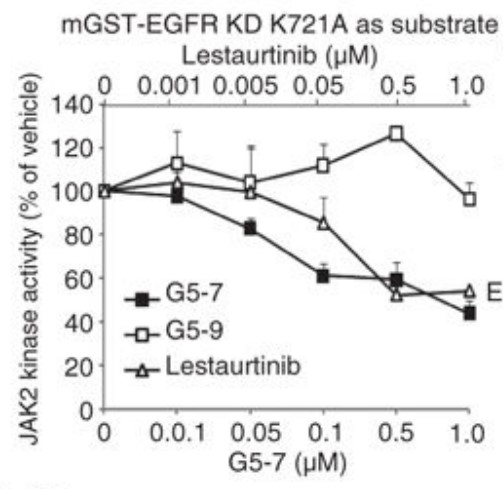
Fig1. Analysis of the in vitro kinase activity of JAK2.
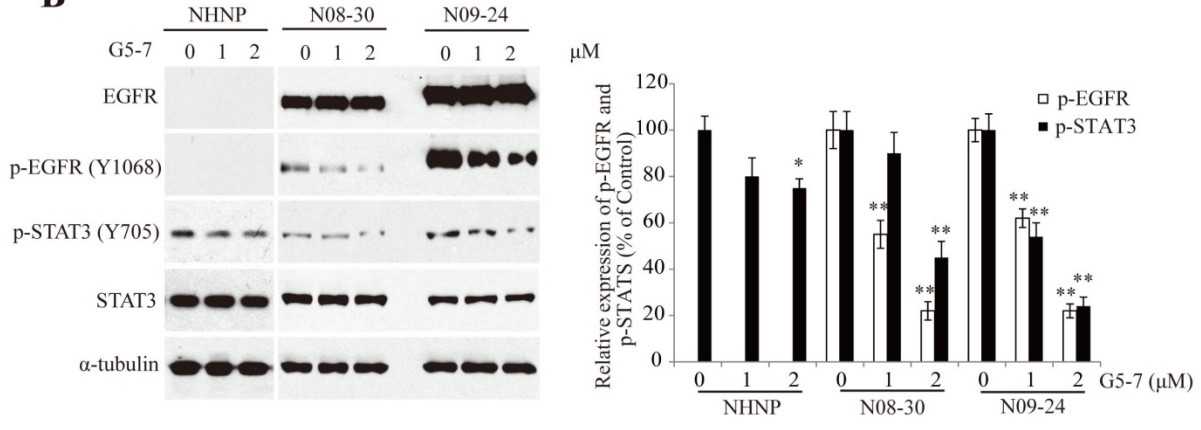
Fig2. G5-7 inhibits EGFR and STAT3 phosphorylation in N09-24 and N08-30 neurospheres.
rhSTAT3, or recombinant human Signal Transducer and Activator of Transcription 3, has several potential applications in the fields of cancer treatment, inflammation, immune response, and regenerative medicine. Some specific applications include:
Tumor Immunity Research: STAT3 protein plays a key role in regulating immune cell function, especially in the tumor immune microenvironment.
Drug Screening and Development: STAT3 is an important drug target, and the study of its inhibitors or degraders contributes to the development of new therapeutic strategies. For example, the ability of SD-36 to act as a STAT3 depressant in reducing STAT3 protein levels in leukemia and lymphoma cells was evaluated in the study.
Disease Diagnosis: The expression level of STAT3 protein may change in some diseases, so it can be used as a diagnostic marker. In breast cancer studies, stat3 protein purified from Sf21 cells transfected with Stat3 gene is used to prepare specific antibodies for early diagnosis of breast cancer.
Protein Function Studies: The recombinant STAT3 protein can be used to study its role in cell signaling, including interactions with other proteins and effects on cell behavior.
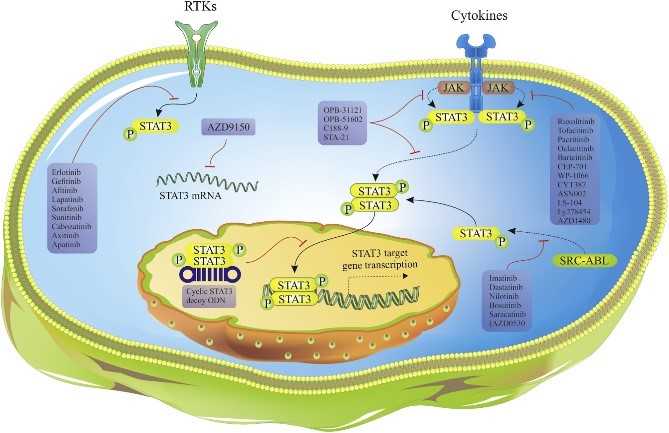
Fig1. Mechanisms of action of different STAT3 inhibitors in market and clinical trials. (Tohid Gharibi, 2020)
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All STAT3 Products
Required fields are marked with *
My Review for All STAT3 Products
Required fields are marked with *



