B7/CD28 Family
Related Symbol Search List
Immunology Background
Background
About B7/CD28 Family
The B7/CD28 family is a group of cell surface molecules involved in regulating immune responses and maintaining immune homeostasis. This family consists of several members, including B7-1 (CD80), B7-2 (CD86), cytotoxic T-lymphocyte antigen 4 (CTLA-4), and programmed death ligand 1 (PD-L1, also known as B7-H1) and PD-L2 (B7-DC, B7-H2). The B7/CD28 family molecules are critical for regulating the balance between immune activation and tolerance. Dysregulation of this pathway can have significant implications for immune-mediated diseases, including autoimmune disorders, chronic infections, and cancer. Therefore, targeting these molecules and their interactions has become an important area of research for developing immunotherapeutic strategies, such as immune checkpoint blockade therapies, which aim to enhance anti-tumor immune responses by blocking inhibitory signals mediated by molecules like CTLA-4 or PD-1.
Co-stimulatory and Co-inhibitory Pathways of the B7/CD28 Family
The B7/CD28 family consists of both co-stimulatory and co-inhibitory pathways that play crucial roles in regulating immune responses. These pathways involve interactions between specific members of the B7/CD28 family and their corresponding receptors. Here's an overview of the co-stimulatory and co-inhibitory pathways within the B7/CD28 family:
| Pathways | Functions | |
|---|---|---|
| Co-stimulatory Pathways | B7-1 (CD80) - CD28 |
|
| B7-2 (CD86) - CD28 |
|
|
| Co-inhibitory Pathways | CTLA-4 - B7-1/B7-2 |
|
| PD-1 - PD-L1/PD-L2 |
|
|
These co-stimulatory and co-inhibitory pathways of the B7/CD28 family help maintain immune homeostasis and regulate the magnitude and duration of immune responses. The balance between co-stimulatory and co-inhibitory signals is crucial for proper immune function, preventing autoimmunity, and avoiding excessive immune activation or tolerance. The understanding of these pathways has led to the development of immunotherapeutic approaches, such as immune checkpoint blockade, to modulate immune responses and treat various diseases, including cancer and autoimmune disorders.
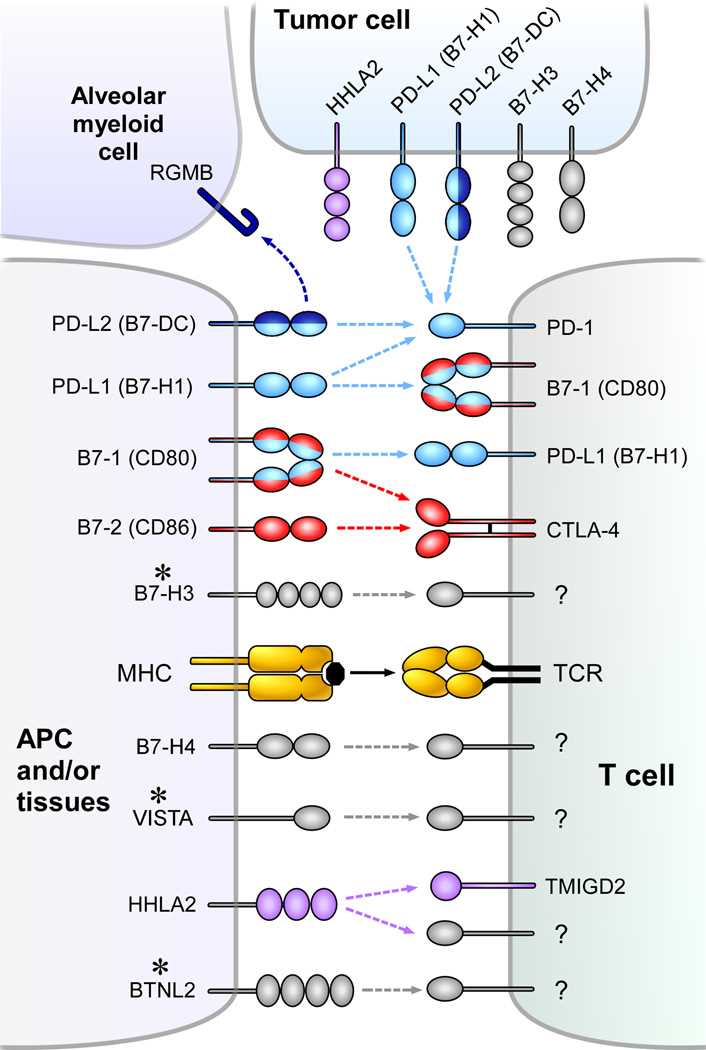 Fig.1 Coinhibitory pathways in the B7-CD28 family. (Schildberg FA, et al., 2016)
Fig.1 Coinhibitory pathways in the B7-CD28 family. (Schildberg FA, et al., 2016)B7/CD28 Family Molecules
The B7/CD28 family consists of several molecules that play critical roles in regulating immune responses. Here are the key members of the B7/CD28 family:
| Key molecule types | Functions |
|---|---|
| B7-1 (CD80) and B7-2 (CD86) |
|
| Cytotoxic T-lymphocyte antigen 4 (CTLA-4) |
|
| Programmed death ligand 1 (PD-L1, B7-H1) |
|
| Programmed death ligand 2 (PD-L2, B7-DC, B7-H2) |
|
The interactions between B7 family molecules (B7-1, B7-2, PD-L1, PD-L2) and their receptors (CD28, CTLA-4, PD-1) play a crucial role in modulating T cell activation, co-stimulation, and immune checkpoint pathways. These interactions regulate the balance between immune activation and tolerance, impacting immune responses in various physiological and pathological conditions.
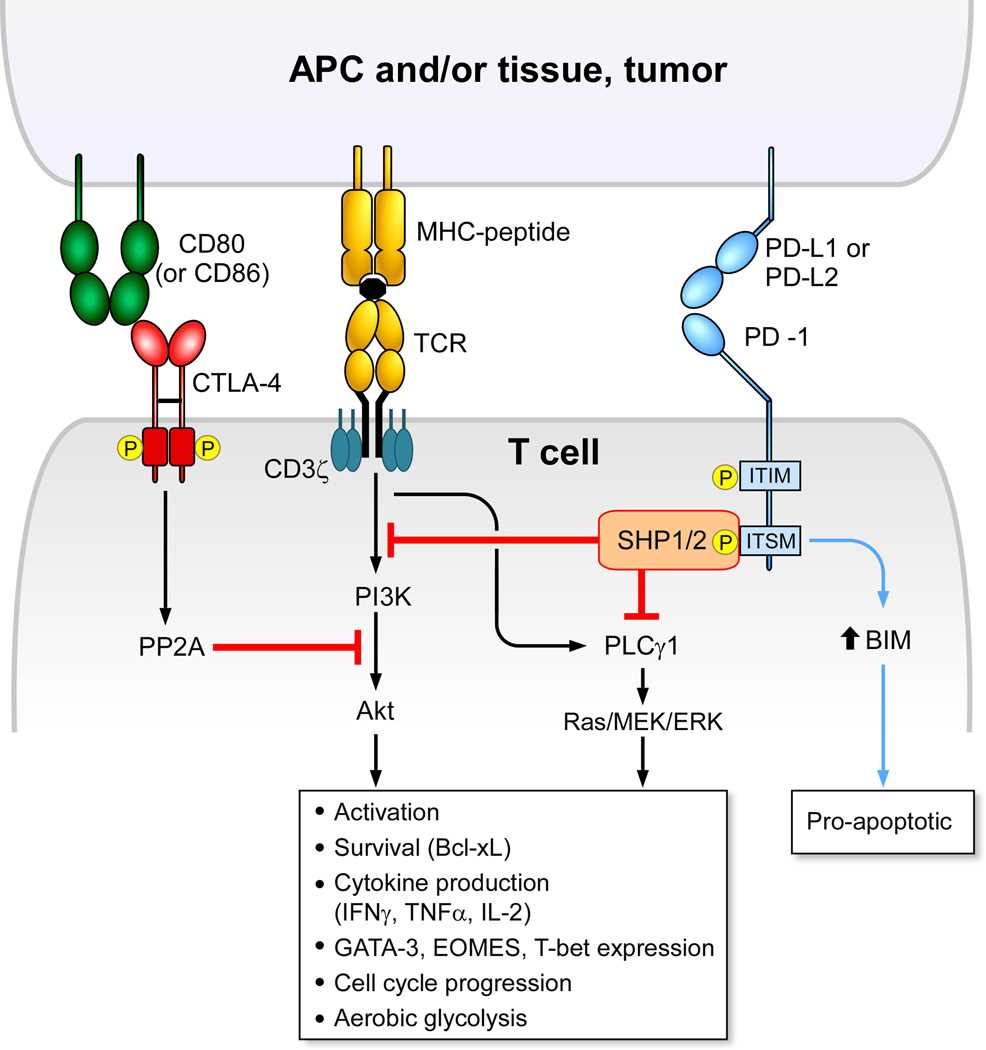 Fig.2 Comparison of intracellular signaling by CTLA-4 and PD-1.(Schildberg FA, et al., 2016)
Fig.2 Comparison of intracellular signaling by CTLA-4 and PD-1.(Schildberg FA, et al., 2016)B7/CD28 Family in Immunomodulation, Tumor Immunity and Autoimmune Diseases
The B7/CD28 family has been extensively studied in the context of immunomodulation, tumor immunity, and autoimmune diseases. Here is an overview of the research progress in these areas:
Immunomodulation
- Co-stimulation: The interaction between B7-1/B7-2 and CD28 provides a co-stimulatory signal necessary for optimal T cell activation. Research has focused on understanding the molecular mechanisms underlying this interaction and its role in regulating immune responses.
- Immune checkpoint blockade: The inhibitory pathways involving CTLA-4, PD-1, and their respective ligands (PD-L1, PD-L2) have been targeted for immune checkpoint blockade therapies. Antibodies blocking CTLA-4 or PD-1/PD-L1 have shown significant clinical success in enhancing anti-tumor immune responses and improving outcomes in various cancers.
- Combination therapies: Researchers are exploring the potential of combining immune checkpoint blockade with other immunomodulatory agents, such as cytokines, immune adjuvants, and targeted therapies, to further enhance anti-tumor immune responses.
Tumor immunity
- Immune evasion mechanisms: Tumor cells often exploit the B7/CD28 family pathways to evade immune surveillance. They can upregulate inhibitory ligands like PD-L1 to suppress T cell responses. Understanding these mechanisms has led to the development of strategies to counteract immune evasion and enhance anti-tumor immunity.
- Biomarkers: Researchers are investigating the expression patterns of B7/CD28 family molecules in tumors as potential biomarkers for predicting response to immune checkpoint blockade therapies. Identifying reliable biomarkers can help personalize treatment decisions and improve patient outcomes.
Autoimmune diseases
- Dysregulation of B7/CD28 family: Alterations in the expression and function of B7/CD28 family members have been implicated in autoimmune diseases. For example, aberrant expression of PD-L1 and PD-L2 has been observed in autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus.
- Therapeutic targeting: Modulating the B7/CD28 family pathways has emerged as a potential therapeutic approach for autoimmune diseases. Blocking the inhibitory signals mediated by CTLA-4 or PD-1/PD-L1 has shown promise in preclinical and clinical studies, offering new avenues for treating these conditions.
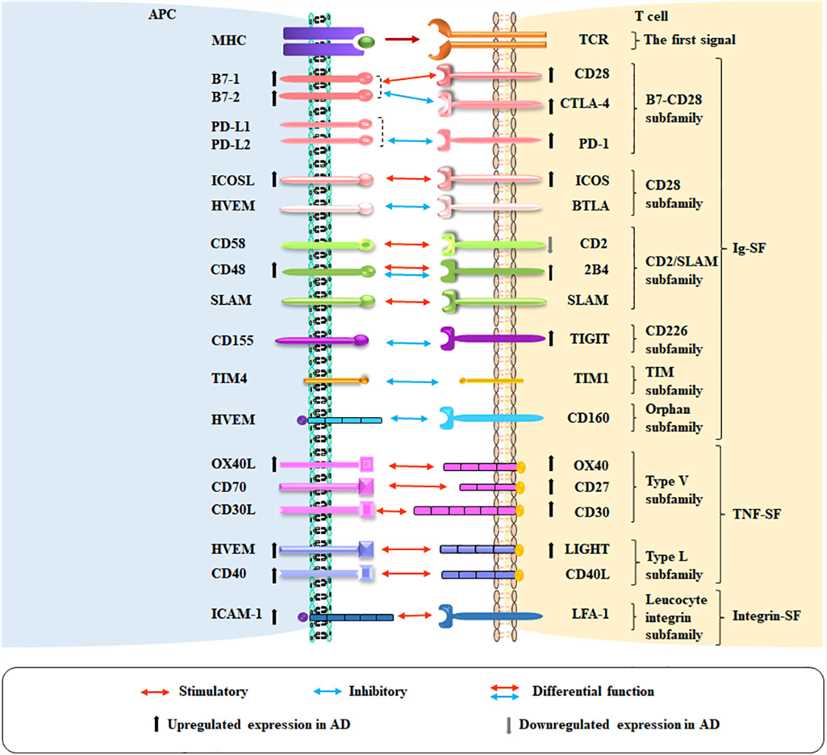 Fig.3 Co-stimulatory and co-inhibitory pathways in atopic dermatitis (AD). (Zheng C, et al., 2023)
Fig.3 Co-stimulatory and co-inhibitory pathways in atopic dermatitis (AD). (Zheng C, et al., 2023)Case Study
Case 1: Tang ZS, Hao YH, Zhang EJ, et al. CD28 family of receptors on T cells in chronic HBV infection: Expression characteristics, clinical significance and correlations with PD-1 blockade. Mol Med Rep. 2016;14(2):1107-1116.
The authors investigated the effects of blocking PD-1 on the levels of co-stimulatory and co-inhibitory receptors. The expression of the CD28 family receptors and intracellular IFN-γ were measured simultaneously in T cells in HLA-A2+ patients with CHB following exposure to anti-PD-L1 or a control antibody. Increased expression of the receptors with PD-1 blockade or isotype antibody treatment are demonstrated by flow cytometric dot plots of peripheral CD4+ T cells from a patient with CHB (A). Following anti-PD-L1 exposure, the expression levels of CD28, ICOS, PD-1 and CTLA-4 were increased in the CD4+ T cells (B). No significant impact on the expression of the CD28 family receptors was observed on the CD8+ T cells following anti-PD-L1 exposure. In addition, following anti-PD-L1 exposure, a significant positive correlation was detected among the HBV DNA titers and the fold changes of PD-1, with the highest fold-change of PD-1 in CD4+ T cells corresponding to viremia levels >106 IU/ml (C).
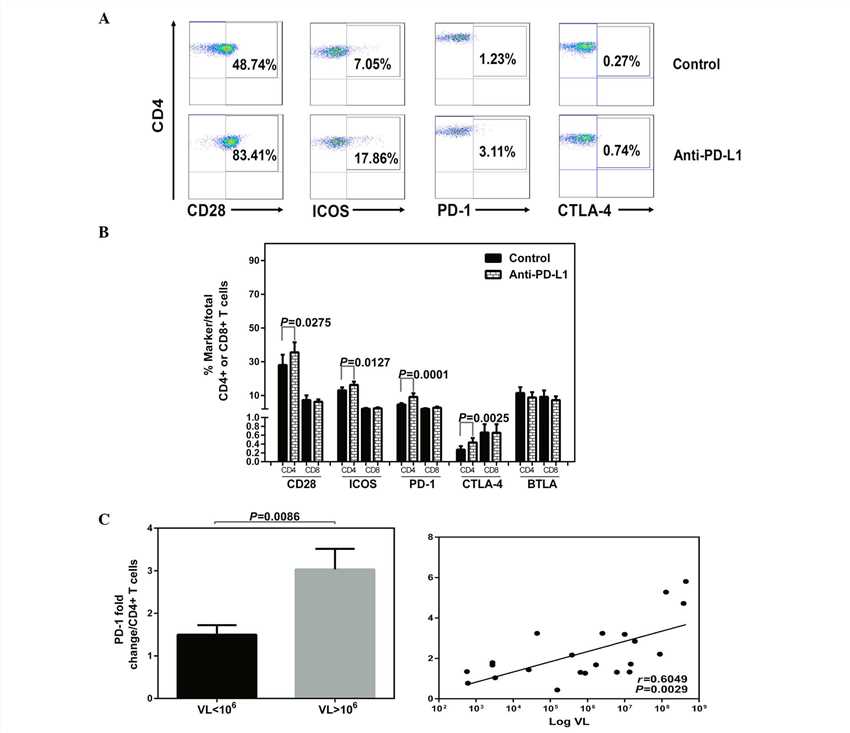 Fig.1 Anti-PD-L1 treatment and expression of CD28 family receptors.
Fig.1 Anti-PD-L1 treatment and expression of CD28 family receptors.Case 2: Deng X, Chen K, Ren J, et al. A B7-CD28 family-based signature demonstrates significantly different prognosis and immunological characteristics in diffuse gliomas. Front Mol Biosci. 2022;9:849723.
The authors performed ESTIMATE and MCP analyses to investigate the relationship between prognostic characteristics and the glioma microenvironment. The authors found that based on the ESTIMATE algorithm, risk scores were positively correlated with immune and stromal scores across all datasets (A-D). Through the MCP approach, the authors further explored the association of genetic features with specific cell populations in the tumor microenvironment. Findings showed that risk scores were significantly correlated with immune cell populations, particularly myeloid dendritic cells, monocyte lineages, and fibroblasts (E, F).
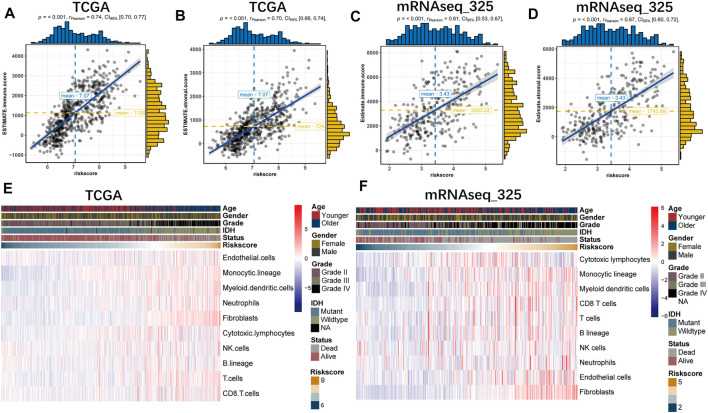 Fig.2 The B7-CD28 family-based signature was tightly associated with immune score (A,B), stromal score (C,D) and infiltrated cells in tumor microenvironment (E,F) in TCGA and mRNAseq_325 cohorts.
Fig.2 The B7-CD28 family-based signature was tightly associated with immune score (A,B), stromal score (C,D) and infiltrated cells in tumor microenvironment (E,F) in TCGA and mRNAseq_325 cohorts.References
- Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44(5):955-972.
- Zheng C, Shi Y, Zou Y. T cell co-stimulatory and co-inhibitory pathways in atopic dermatitis. Front Immunol. 2023;14:1081999.
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116-126.
- Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128-143.

