Human Synthetic Protein Production in Mammalian Cell Lines
Creative BioMart offers Customized Production of Human Synthetic Proteins in Mammalian Cell Lines, featuring enhanced glycosylation and increased biological activity. Our proprietary technology involves stable transfection with human sialyltransferases, resulting in proteins with human-like glycosylation patterns, improved bioavailability, and reduced immunogenicity—key advantages for therapeutic and research applications. This innovative approach not only boosts therapeutic efficacy but also reduces production and storage costs. Whether you're studying protein interactions, channel activity, or signaling pathways, our mammalian-based expression systems provide high-quality, customized protein solutions tailored to your scientific and clinical needs.
Background: Why Mammalian Cell Lines for Human Synthetic Proteins
Human synthetic proteins produced in mammalian cell lines are a cornerstone of modern biotechnology and pharmaceuticals. These proteins are used for a wide range of applications, including therapeutic drugs, vaccines, and diagnostic tools. Mammalian cell lines, particularly Chinese Hamster Ovary (CHO) cells and Human Embryonic Kidney (HEK) 293 cells, are preferred for producing complex human proteins due to their ability to perform post-translational modifications (PTMs) and ensure proper protein folding.
Production Process
- Gene Transfection: The gene of interest is transfected into the mammalian cells using methods such as polyethyleneimine (PEI) or calcium phosphate.
- Protein Expression and Purification: Once the gene is expressed, the proteins are secreted into the culture medium. The proteins are then purified using techniques such as chromatography. The yield and quality of the proteins are critical factors in the efficiency of the production process
At Creative BioMart, we've developed a novel platform to further enhance this process: stable transfection of mammalian cells with human sialyltransferases, the key enzymes involved in protein glycosylation. This method allows us to produce synthetic human proteins with glycosylation profiles that closely mimic native human proteins, improving both serum half-life and functional activity.
This approach offers significant benefits:
- Increased therapeutic activity through improved protein stability and bioavailability
- Reduced immunogenicity, minimizing potential side effects
- Lower production and storage costs due to higher protein stability
Whether your application is therapeutic, diagnostic, or exploratory research, Creative BioMart’s technology delivers custom proteins with enhanced performance and human compatibility.
Overview of Our Mutagenesis Service
Service Procedure

Service Offerings: Tailored Mutagenesis Options
-
Cell Line Options
CHO, HEK293, and other mammalian platforms
-
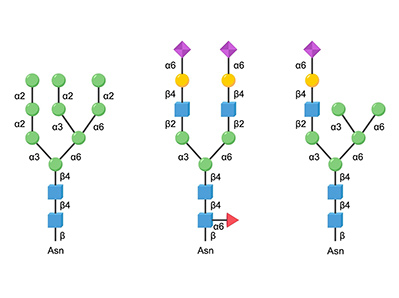
Glycosylation Control
Human-like sialylation patterns via engineered sialyltransferases
-

Customization
Tailored expression levels, tags, modifications, and formulation options
Key Benefits of Partnering with Us for Mammalian Cell Protein Expression
- Human-like Glycosylation: Achieve superior protein humanization for better performance in clinical and research models.
- Higher Therapeutic Activity: Extended serum half-life and bioavailability result in more potent protein therapeutics.
- Reduced Immunogenicity: Lower risk of immune responses ensures better safety and efficacy.
- Cost-Effective Production: Stable proteins mean longer shelf life, reduced waste, and lower storage costs.
- Proven Track Record: Decades of expertise in cell line development and custom protein production.
- Fully Customized Service: Every project is tailored—from gene design to final protein format—to fit your specific goals.
Proven Success in Human Synthetic Protein Projects Using Mammalian Expression Platforms
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Improved efficacy of recombinant α-galactosidase via enhanced glycosylation
Sohn et al., 2013. doi:10.5483/BMBRep.2013.46.3.192
Researchers successfully expressed recombinant human α-galactosidase A (GLA) in Chinese hamster ovary (CHO) cells for enzyme replacement therapy in Fabry disease, achieving high productivity. The initial product lacked sufficient terminal sialic acids, resulting in smaller size and altered charge compared to agalsidase β, a commercial therapeutic. Since sialylation alone was insufficient, a combined enzymatic modification using galactosyltransferase and sialyltransferase with sugar substrates was developed to enhance glycosylation. This optimized process yielded GLA with properties closely matching agalsidase β, including improved sialic acid content and in vivo efficacy, demonstrating the importance of glycoengineering in mammalian cell systems for therapeutic protein humanization.
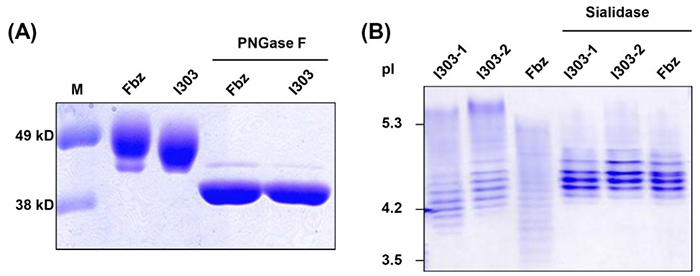
Figure 1. Comparison of the sizes and charges of agalsidase β and I303. (A) Sizes of agalsidase β (Fbz; Fabrazyme) and I303 were compared in SDS-PAGE before and after deglycosylation using PNGase F digestion. (Sohn et al., 2013)
Case 2: Optimizing HCV E1E2 vaccine production via PPI mapping in CHO cells
Min Wu et al., 2025. doi:10.1101/2025.05.20.655202
Researchers tackled the challenge of low-yield recombinant production of the HCV sE1E2 vaccine protein in Chinese hamster ovary (CHO) cells. Using a multi-omics approach—combining Biotinylation by Antibody Recognition (BAR) to map protein-protein interactions and RNA-Seq—they identified secretory pathway proteins that enhance sE1E2 secretion. Overexpression of key interactors, CUL4A and YWHAH, significantly improved yields in glycoengineered CHO (geCHO) cells. This study highlights the power of integrated omics and cell line engineering to enhance human synthetic protein production in mammalian systems, offering a promising strategy for improving the affordability and accessibility of complex biologics like vaccines.
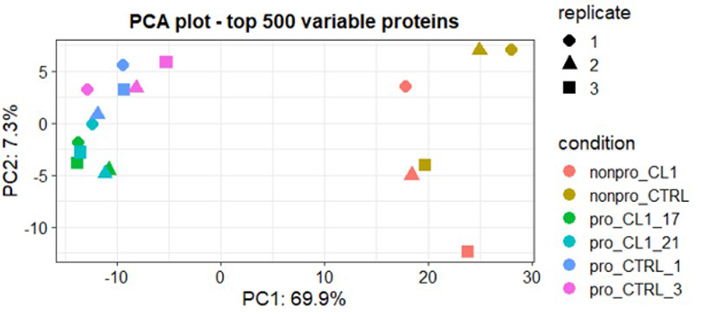
Figure 2. Principal Component Analysis (PCA) of the normalized proteomic dataset demonstrated clear separation between nonproducer (parental) cell lines and E1E2 producer clones. Principal component 1 (PCA1) captured 70% of the total variation, effectively distinguishing between the two cell line types. (Min Wu et al., 2025)
What Our Clients Say About Our Mammalian Cell Protein Production Services
“We needed a glycosylated version of a human cytokine with a His-tag for our receptor binding assays. Creative BioMart delivered exactly what we needed using their CHO expression platform. The glycosylation profile was impressively human-like, and the protein showed excellent activity and stability. Their technical team provided outstanding support throughout.”
— Senior Scientist | Biotech R&D Division
“For a kinase isoform study, we required a FLAG-tagged synthetic human protein expressed in HEK293 cells with specific post-translational modifications. Creative BioMart customized everything flawlessly—from construct design to purification. The expression level and yield exceeded expectations, and the protein was functionally validated in our in-house assays. Their attention to detail is top-tier.”
— Director of Protein Engineering | Pharmaceutical Company
“We collaborated with Creative BioMart on a project involving a glycoprotein that loses activity when expressed in E. coli . Their mammalian cell system with engineered sialyltransferases produced a version with much higher bioactivity and a longer serum half-life. This allowed us to proceed with in vivo testing without delay. Highly recommended for functional protein studies.”
— Project Manager | Academic Drug Discovery Center
“We needed a biotin-tagged human transporter protein with authentic glycosylation for use in a high-sensitivity diagnostic platform. Creative BioMart used a stable CHO cell line to produce the protein with high purity and structural integrity. Their team worked with us to optimize buffer conditions, and the final product integrated seamlessly into our assay. Great value and excellent communication!”
— Research Lead | Diagnostics Development Company
Frequently Asked Questions–Human Synthetic Protein Production in Mammalian Cells
-
Q: What are the main advantages of producing synthetic human proteins in mammalian cell lines?
A: Our mammalian expression system—enhanced with human sialyltransferases—produces proteins with authentic human-like glycosylation patterns, leading to:- Increased bioactivity and serum half-life
- Reduced immunogenicity
- Improved protein stability and lower production/storage costs
-
Q: Can you customize the protein tag, expression system, or purification method?
A: Absolutely. We offer full customization, including:- Choice of expression host (e.g., CHO, HEK293)
- Custom affinity tags (e.g., His, FLAG, biotin)
- Purification options based on your downstream needs
- Buffer optimization for storage and stability
-
Q: How do your proteins compare with those produced in E. coli or insect cells?
A: Unlike E. coli or insect systems, our mammalian cell lines enable proper glycosylation and folding of complex human proteins. This makes them more:- Functionally active
- Stable in serum
- Biologically relevant for therapeutic or structural studies
-
Q: What types of proteins have you successfully produced?
A: Our technology has been used to express a wide range of synthetic human proteins, including:- Ion channel modulators
- Kinase isoform-selective targets
- Protein-protein interaction complexes
- Proteins for RNA interference and signal transduction studies
Resources
Related Services
- Mammalian Expression Systems
- Protein Glycosylation Labeling Service
- Protein Expression and Purification Services
- Protein Engineering Services
- Bacterial Expression Systems (E. coli / Bacillus)
- Baculovirus-Insect Cell Expression
- Protein Interaction Service
- Ion Channel Screening Assays
- Protein Pathway Profiling
Related Products
References:
- Min Wu MY, Rocamora F, Robinson CM, et al. Enhanced production of HCV E1E2 subunit vaccine candidates via protein-protein interaction identification in glycoengineered CHO cells. Published online May 23, 2025. doi:10.1101/2025.05.20.655202
- Sohn Y, Lee JM, Park HR, Jung SC, Park TH, Oh DB. Enhanced sialylation and in vivo efficacy of recombinant human α-galactosidase through in vitro glycosylation. BMB Reports. 2013;46(3):157-162. doi:10.5483/BMBRep.2013.46.3.192
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

