Membrane Protein Extraction
High-quality membrane protein extraction is fundamental for understanding cellular signaling, transport, energy conversion, and interactions occurring at the cell boundary. Owing to their intrinsic hydrophobicity and tight association with lipid bilayers, membrane proteins present unique challenges in enrichment, solubilization, purification, and downstream analysis. Creative BioMart provides comprehensive, optimized Membrane Protein Extraction Services, combining advanced solubilization chemistry, customizable enrichment workflows, and rigorous analytical validation. Equipped with more than 100 solubilization agents, state-of-the-art separation platforms, and extensive expertise in handling complex membrane proteomes, we deliver highly enriched, stable, and functional membrane protein samples tailored for proteomics, structural biology, and drug discovery applications.
Background: Significance of Membrane Protein Extraction
Membrane proteins perform some of the most essential biological functions within the cell. They enable nutrient import, waste export, ion exchange, signal transduction, cell–cell interactions, membrane fusion, and environmental sensing. This functional significance makes them indispensable targets for biochemical investigation and pharmacological development—indeed, more than 60% of therapeutic drugs target membrane-associated proteins such as GPCRs, ion channels, and transporters.
However, the biochemical complexity of membrane proteins has long presented barriers to comprehensive characterization. Their amphipathic nature, low expression levels, tight association with lipid bilayers, and—frequently—large hydrophobic transmembrane regions make isolation difficult without damaging structure or function. Two major issues consistently challenge classical workflows:
- Enrichment: Isolating membrane proteins from cytosolic or organellar contaminants while preserving their integrity.
- Solubilization: Maintaining native conformation after extraction, especially when hydrophobic segments resist aqueous environments.
To address these challenges, innovative methodologies have been developed. Membrane enrichment may rely on differential or density gradient centrifugation, solvent-based delipidation, carbonate stripping, aqueous polymer partitioning, or cationic silica binding. Meanwhile, solubilization strategies have expanded from traditional detergents to ionic liquids, chaotropes, zwitterionic surfactants, and nanodisc reconstitution systems that mimic natural bilayers. These advances not only improve yield and purity but also enable more accurate and representative proteome coverage.
Yet, even after successful extraction, maintaining protein stability and ensuring compatibility with downstream techniques—such as LC-MS/MS, cryo-EM, SDS-PAGE, 2-D gels, or functional assays—requires careful purification and fractionation. Methods performed at the peptide level provide high solubility and recovery, while protein-level approaches enhance detection of low-abundance targets by reducing complexity.
With decades of combined expertise and a broad portfolio of specialized chemical agents, Creative BioMart offers a refined and validated workflow for membrane protein extraction—tailored to each sample type and research objective.
Membrane Protein Extraction: What We Offer
Creative BioMart provides fully customized Membrane Protein Extraction Services designed to handle the most complex and challenging membrane systems. Our offerings include:
-
Comprehensive Membrane Protein Enrichment Strategies
Including sucrose gradients, aqueous polymer two-phase separation, high-salt/high-pH extraction, solvent delipidation, and carbonate stripping.
-
More than 100 Solubilization Agents
Selected based on protein type, sample source, and downstream application.
-
Customizable Digestion Options
Including in-solution digestion, filter-aided sample preparation (FASP), and optimized detergents compatible with MS.
-
Protein-level and Peptide-level Purification Workflows
Ensuring maximal coverage and stability.
-
High-Throughput Quality Control
Including SDS-PAGE, Western blotting, spectrophotometric assays, LC-MS/MS profiling, and purity assessment.
Supported Membrane Protein Categories
Our service is suitable for numerous membrane protein classes, including:
We support samples from cell lines, tissues, microbial cells, plants, and difficult-to-lyse systems.
Service Workflow
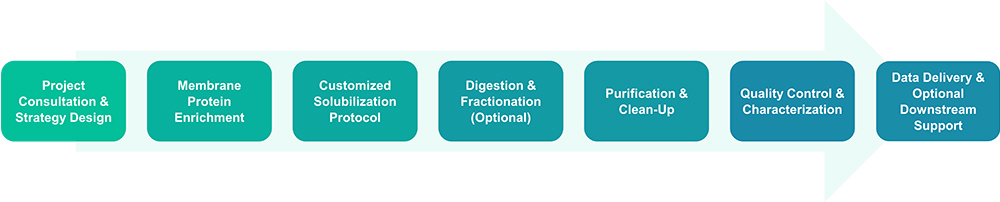
Service Features
-
Membrane Protein Enrichment Techniques
We tailor enrichment methods to maximize yield and reduce contamination. Common techniques include:
- Sucrose gradient centrifugation for delicate eukaryotic membranes.
- Aqueous two-phase partitioning, ideal for plant and microbial systems.
- Cationic colloidal silica coating to isolate plasma membranes efficiently.
- Solvent delipidation and carbonate stripping to remove excess lipids while maintaining protein usability.
- High-salt or high-pH extraction to release peripheral proteins.
Each method is selected based on biological source, membrane composition, and downstream biochemical requirements.
-
Solubilization Strategies
Solubilization agents are chosen to maintain the native conformation when necessary or to fully denature proteins for proteomic applications:
- Non-denaturing agents: DDM, MNG, Triton™ X-100/114, digitonin.
- Denaturing agents: SDS, PPS, urea, ionic liquids.
- Nanodisc platforms: used to reconstitute membrane proteins into lipid bilayers, ideal for structural studies.
Our deep understanding of solvent–protein interactions allows us to optimize solubilization while minimizing loss of native activity.
-
Purification and Fractionation
Purification strategies are performed at either the protein or peptide level:
- Protein-level purification to enhance detection of low-abundance proteins.
- Peptide-level fractionation (e.g., SCX, HILIC, high-pH reversed-phase) for improved MS performance.
Additional clean-up may include precipitation, desalting, and lipid removal.
-
Analytical Quality Control
QC is tailored to each project and may include:
- SDS-PAGE and Western blotting
- Nanodrop and BCA quantification
- LC-MS/MS profiling
- Purity and contamination analysis
- Functional activity tests (for selected protein classes)
What Sets Us Apart
- Extensive Solubilization Library: With over 100 solubilization agents, we can adapt protocols to even the most recalcitrant membrane proteins, ensuring optimal stability and recovery.
- Advanced Membrane Enrichment Expertise: Our team has deep experience with sucrose gradients, polymer partitioning, silica-based enrichment, and other specialized membrane isolation systems.
- Custom-Built Workflows: Every project is tailored from the ground up—no generic one-size-fits-all protocols. We optimize for your protein class, species, and analytical goals.
- Comprehensive QC and Analytical Support: From electrophoresis to high-resolution mass spectrometry, we ensure clarity and confidence in every data point delivered.
- Compatibility with Diverse Downstream Applications: Our extracted membrane proteins are suitable for proteomics, structural biology, antibody generation, drug screening, and biochemical assays.
- One-Stop, High-Reliability Service: From project design to data interpretation, we provide an integrated workflow backed by decades of expertise in protein chemistry and membrane biology.
Membrane Protein Extraction: Case Studies
Case 1: Membrane protein extraction in salt-stress soybean research
Rehman et al., 2022. doi:10.3390/ijms232113270
To investigate how soybean responds to salinity, membrane proteins were extracted from roots and leaves of the salt-sensitive cultivar C08 germinated under NaCl stress. The workflow enabled the identification of 972 membrane proteins, including receptor kinases, ion channels, calcium sensors, ABA receptors, and transporters. Tissue-specific enrichment revealed endocytosis and lipid-metabolism–related proteins in roots, while leaves showed enrichment in phagosome, spliceosome, and SNARE-interaction pathways. Label-free quantitation identified 129 differentially expressed proteins, along with NaCl-induced vesicle, mitochondrial, and chloroplast-associated membrane proteins. Validation with RNA-seq data highlighted key solute-transport and stress-sensing mechanisms in soybean salt responses.
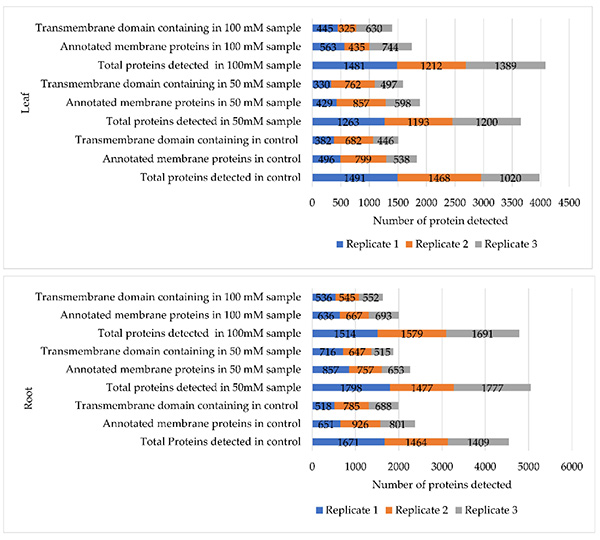
Figure 1. Comparisons of the numbers of total identified proteins against the numbers of transmembrane domain-containing proteins among all three biological replicates in both leaf (upper panel) and root tissues (lower panel). (Rehman et al., 2022)
Case 2: Optimizing membrane protein extraction for deep proteomic profiling
Kongpracha et al., 2022. doi:10.1016/j.mcpro.2022.100206
To overcome the challenges posed by low abundance and hydrophobicity of membrane proteins, a comparative extraction study was performed using HEK293T cells. Multiple enrichment strategies—including ultracentrifugation, urea washing, and alkaline treatment—were evaluated prior to LC–MS/MS analysis. Urea washing proved most effective, doubling the total number of identified membrane proteins and increasing multispanning proteins such as SLC transporters, ABC transporters, and GPCRs nearly sixfold. This optimized workflow enabled accurate profiling of amino acid transport systems and revealed additional membrane protein complexes. The protocol, built entirely on standard biochemical procedures, provides a robust approach for in-depth membrane proteome characterization across diverse sample types.
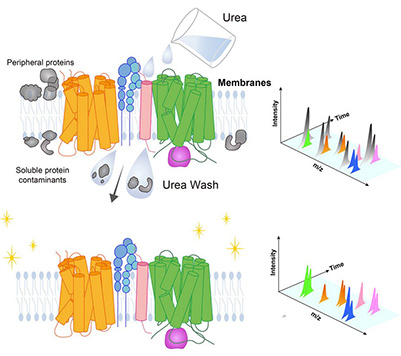
Figure 2. Graphic abstract: Simple but efficacious enrichment of integral membrane proteins and their interactions for in-depth membrane proteomics. (Kongpracha et al., 2022)
Membrane Protein Extraction: Frequently Asked Questions
“We approached Creative BioMart with a notoriously difficult Class B GPCR that resisted every extraction approach we tried in-house. Their team optimized a detergent-screening workflow and delivered a membrane prep with excellent purity and stability. The extracted GPCR performed beautifully in downstream LC-MS/MS and ligand-binding assays, giving our early discovery program a major boost.”
— Senior Scientist, Drug Discovery Division | Global Pharmaceutical Company
“Our lab needed high-quality membrane fractions from human liver tissue for a metabolic disorder study. Creative BioMart handled the complex samples with remarkable precision, using gradient enrichment and MS-compatible clean-up to preserve even low-abundance transporters. The resulting dataset showed outstanding coverage across multiple transporter families and accelerated our biomarker validation work.”
— Head of Proteomics Core | Leading Academic Medical Center
“We relied on Creative BioMart to extract functional ion channels from engineered HEK293 lines for our electrophysiology screening panel. Their gentle extraction strategy preserved channel activity, and the nanodisc-based stabilization they recommended dramatically improved our signal consistency. This partnership helped us launch a more robust screening platform ahead of schedule.”
— Director of Assay Development | Biotech Focused on Neurological Diseases
“Our team needed purified membrane proteins from anaerobic bacteria to evaluate stress-response pathways relevant to large-scale fermentation. Creative BioMart delivered clean, highly enriched fractions despite the organism’s tough cell wall and sensitivity to oxygen. Their extraction enabled us to identify key redox enzymes that we later engineered to improve fermentation efficiency by 18%.”
— Principal Investigator | Industrial Microbiology & Fermentation Company
Nuclear Protein Extraction Service: Frequently Asked Questions (FAQs)
-
Q: What types of membrane proteins can Creative BioMart extract?
A: We work with an exceptionally broad range of membrane proteins, including GPCRs, ion channels, transporters, membrane-bound enzymes, adhesion proteins, and fusion proteins. Thanks to our extensive library of over 100 solubilization agents and multiple enrichment platforms, we routinely handle even highly hydrophobic or low-abundance membrane proteins from diverse organisms and tissue types. -
Q: How do you determine the best solubilization agent for my project?
A: We evaluate your sample type, membrane composition, target protein class, and downstream applications to select the most compatible solubilization strategy. Our portfolio includes non-denaturing detergents, denaturants, ionic liquids, and nanodisc systems. We may also perform small-scale optimization to ensure maximum recovery and structural stability of your target proteins. -
Q: Do you offer membrane protein extraction compatible with mass spectrometry?
A: Absolutely. Our workflows are fully MS-compatible. Depending on your analytical goals, we provide detergent-free clean-up, peptide-level fractionation, optimized digestion protocols, and high-throughput QC. These approaches ensure excellent membrane proteome coverage and clear identification of low-abundance targets. -
Q: Can you help with downstream analysis after extraction?
A: Yes—we offer optional services such as LC-MS/MS profiling, SDS-PAGE/Western blot verification, functional assay compatibility assessment, and data interpretation. Many customers rely on our end-to-end support to streamline their research workflow from extraction through characterization. -
Q: How do you ensure high purity of the membrane fractions?
A: Purity is controlled through a combination of optimized enrichment procedures—such as sucrose gradients, polymer partitioning, or silica-based surface extraction—and rigorous QC. We monitor membrane purity using electrophoresis, marker protein checks, MS-based evaluation, and quantitative analysis to ensure minimal cytosolic or organellar contamination. -
Q: Can membrane proteins be preserved in their native, functional state?
A: Yes. For applications requiring activity retention, we use gentle non-ionic detergents, mild purification strategies, and—in many cases—nanodisc reconstitution to maintain native protein conformation. This approach is ideal for functional studies, structural biology, and drug-binding experiments. -
Q: What sample types do you accept?
A: We support cultured cells, tissues, microbial systems, plant samples, and other complex biological materials. Our team adjusts the enrichment and solubilization protocols to suit the specific challenges of each sample type, ensuring high-quality extraction regardless of biological origin. -
Q: How do I know if your workflow is suitable for my difficult or low-yield target proteins?
A: Our scientists perform a detailed technical evaluation before project initiation. We assess extraction feasibility, discuss solubilization options, and recommend the most effective strategies. For challenging targets, we can conduct pilot-scale tests to verify recovery and stability, reducing risk and ensuring confidence in final project outcomes.
Other Resources
Related Services
- Protein Extraction Services
- Organelle Protein Extraction
- Nuclear Protein Extraction
- Cytoplasmic Protein Extraction
- Membrane Proteins Expression and Purification
- Membrane Protein Screening
- GPCR Screening Assays
- Ion Channel Screening Assays
- Transporter Screening Assays
- Detergent Micelle Platform
Related Products
References:
- Kongpracha P, Wiriyasermkul P, Isozumi N, Moriyama S, Kanai Y, Nagamori S. Simple but efficacious enrichment of integral membrane proteins and their interactions for in-depth membrane proteomics. Molecular & Cellular Proteomics. 2022;21(5):100206. doi:10.1016/j.mcpro.2022.100206
- Rehman HM, Chen S, Zhang S, et al. Membrane proteomic profiling of soybean leaf and root tissues uncovers salt-stress-responsive membrane proteins. IJMS. 2022;23(21):13270. doi:10.3390/ijms232113270
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

