phospho-Serine/phospho-Threonine Binding Proteins
Related Symbol Search List
Immunology Background
About Phospho-Serine / Phospho-Threonine Binding Proteins
Phospho-serine / phospho-threonine binding proteins are a class of proteins that play key roles in various cellular processes. These proteins specifically recognize and interact with phosphorylated serine (pSer) and threonine (pThr) residues within target proteins. By binding to these phosphorylated residues, they regulate protein function, mediate protein-protein interactions, and promote signal transduction pathways.
The first phosphoserine/threonine-binding molecules that were identified were members of a family of dimeric proteins called 14-3-3 that were first identified as abundant polypeptides of unknown function in the brain; they were later identified as activators of tryptophan and tyrosine hydroxylase and as inhibitors or activators of PKCs. Mammalian cells contain 7 distinct 14-3-3 gene products (denoted β, γ, e, η, σ, t, and ζ), while plants and fungi contain between 2 and 15. Several of the mammalian 14-3-3 isotypes are subject to phosphorylation, although the role that phosphorylation plays in the 14-3-3 function remains speculative.
Functions of Phospho-Serine / Phospho-Threonine Binding Proteins
- Phosphoprotein Analysis
phospho-serine / phospho-threonine binding proteins are widely used in phosphoprotein analysis and phosphoproteomics. They enable selective enrichment and purification of phosphorylated proteins from complex biological samples, providing insight into protein phosphorylation kinetics and cell signaling pathways.
- Signal Transduction Studies
Phosphorylation-mediated signaling is a fundamental process in cellular signaling cascades. phospho-serine / phospho-threonine binding proteins are important tools for studying the regulation and modulation of protein phosphorylation events in signal transduction pathways. They help identify key protein-protein interactions, study kinase-substrate relationships, and decipher phosphorylation-dependent cellular processes.
- Drug Development
Dysfunction of phosphorylation events is commonly associated with a variety of diseases, including cancer, neurological disorders, and metabolic disorders. Understanding the mechanisms of protein phosphorylation and the involvement of phospho-serine / phospho-threonine binding proteins can help in the development of targeted therapeutic strategies. Creative BioMart supports drug discovery efforts by providing customized protein production, functional assays, and binding studies related to phospho-serine / phospho-threonine binding proteins.
Mechanism of Action of Phospho-Serine/phospho-Threonine Binding Proteins
Phospho-serine / phospho-threonine binding proteins contain conserved structural domains such as the WD40 repeats, the Polo-Box structural domain (PBD), the 14-3-3 structural domain, and the forkhead-associated (FHA) structural domain. These domains recognize and bind phospho-serine / phospho-threonine residues on target proteins through specific interactions. This binding induces conformational changes in the target proteins that modulate their function, stability, intracellular localization, or interaction with other molecules. In addition, phospho-serine / phospho-threonine binding proteins can act as scaffolds to bring together multiple signaling proteins to facilitate signal propagation and amplification.
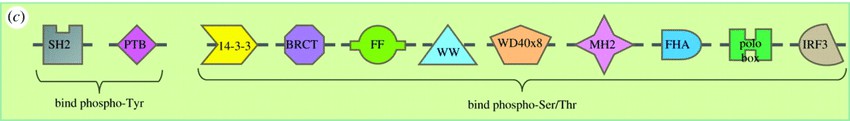 Fig.1 Phospho-tyrosine and phospho-serine/threonine binding domains. (Panizza E., et al., 2017)
Fig.1 Phospho-tyrosine and phospho-serine/threonine binding domains. (Panizza E., et al., 2017)
Research Tools for Phospho-serine / Phospho-threonine Binding Proteins
Phospho-serine / phospho-threonine binding proteins are key players in cellular signaling networks that contribute to a variety of cellular processes and disease mechanisms. Creative BioMart provides resources and services that can facilitate research and development in this area, improve our understanding of phosphorylation-dependent signaling pathways, and help develop novel therapeutic interventions. Based on our listing of phospho-serine / phospho-threonine binding proteins, click to view all related molecules/targets and research reagents. Please get in touch with us with any questions or requests.
References:
- Panizza E. Characterizing cancer cell signaling at the protein level: from targeted to proteome and phosphoproteome ide analyses[J]. 2017.
- Yaffe M B, Aeh E. Phosphoserine/threonine-binding domains [Review][J]. Current Opinion in Cell Biology, 2001(2):13.

