Protein G Agarose High Flow Resin
| Cat.No. : | COP28 |
| Availability | January 27, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Tag : | Non |
| PRODUCTINFORMATION : | Product Overview: Protein G Agarose High Flow Resin belongsto the resins family for capture of monoclonal antibodies (MAbs), and isavailable with different pack sizes for easy Mabs purification, processdevelopment and scale up. Immunoprecipitation studies with Protein G AgaroseHigh Flow are also commonly used to purify proteins or protein complexes.Description: Protein G AgaroseHigh Flow Resin, with rProtein G coupled to high flow Agarose matrix, is avery popular affinity resin for poly and monoclonal antibody/fragmentspurification and isolation of immune complex. Protein G exhibit bindingspecificities that complement Protein A media and binds to the Fc region ofIgG from a variety of mammalian species. Compared to protein A, protein Gbinds more strongly to polyclonal IgG from cow, sheep and horse. Furthermore,unlike protein A, Protein G Agarose High Flow Resin can be used successfullyto capture polyclonal rat IgG, human IgG3 and mouse IgG1. Moreover, Protein GAgarose High Flow resin is extremely useful in immunoprecipitation (IP)procedures successfully.Features: Binding specificities that complementProtein A media. First choice for purification of whole IgG from serum orascites at high yield and high purity. Suitable for purification of poly ormonoclonal antibodies of different species and subclasses for its broadbinding specificity. No albumin binding domain and avoid undesirable interactionswith albumin. Single point attachment coupling chemistry gives better ligandaccessibility for higher binding capacitySpecifications:Note:Determined at 10% breakthrough at 4min residence time. Delivered at roomtemperature, and recommended long-term storage at 2-8 °C. |
| INSTRUCTIONS FORPURIFICATION : | 1. Column packing:ProteinG Agarose High Flow is supplied as a suspension in 20% ethanol. Decant the20% ethanol solution and exchange it with water or other packing bufferrequired before use. Then follow the procedures below:(1). Equilibrate all material to the temperatureat which the purification will be performed. Assemble the column (and packingdevice, if necessary).(2). Remove air from the column dead spaces byflushing the end-piece and adapter with packing buffer. Make sure no air hasbeen trapped under the column net. Close the column outlet leaving the netcovered with packing buffer.(3). Resuspend the medium stored in itscontainer by shaking (avoid stirring the sedimented medium). Mix the packingbuffer with the medium to form a 50% to 70% slurry (sedimented bedvolume/total slurry volume = 0.5 to 0.7).(4). Pour the homogeneous slurry into the columnin a single continuous motion. Pouring the slurry down a glass rod heldagainst the column wall will help to minimize the introduction of airbubbles.(5). If using a packing device, immediately fillthe remainder of the column and packing device with packing buffer. Mount theadapter or lid of the packing device and connect the column to a pump. Avoidtrapping air bubbles under the adapter or in the inlet tubing.(6). Open the bottom outlet of the column andturn on the pump to run at the desired flow rate. Ideally, Protein G resin ispacked at a constant pressure of approximately 1 bar (0.1 MPa). If thepacking equipment does not include a pressure gauge, use a packing flow rateof approximately 400 cm/h (10 cm bed height, 25°C, water as packing buffer).If the recommended pressure or flow rate can not be obtained, use the maximumflow the pump can deliver.(7). When the bed height has stabilized, markthe compressed bed height and close the bottom outlet and stop the pump.(8). If usinga packing device, disconnect the packing device and mount the adapter to the column.(9). With theadapter inlet open, push the adapter down, approximately 2 mm below previouscompressed bed height, and the packing buffer will flush the adapter inlet.Close the adapter inlet.(10). Thecolumn is now ready to use for antibody purification.Note: Do notexceed 75% of the packing flow rate in subsequent chromatographicpurification procedures.2. Purification2.1 Binding AffinityProtein Gligand has versatile IgG binding specificity and affinity, and offers highselectivity and resolution for efficient capture of antibodies from anybiological fluid or cell culture medium. Thus, the potential applications ofprotein G include practically almost all of the current and projectedapplications of protein A. However, Protein G has different IgG bindingspecificities dependent on the origin of the IgG. Binding characteristics ofProtein G ligand are summarized in Table 2, which can be used as generalguide for affinity separation media selection of antibody purification.Protein G has a low affinity site for the Fab region, therefore, Protein GAgarose High Flow can sometimes be used for purification of Fab and F(ab')2fragments.Thedynamic binding capacity is a function of the sample residence time. It isnecessary to use appropriate linear flow rate during sample application toensure that residence time is in 4 to 8 min range at optimal column height of5 to 20 cm. The residence time is equal to the packed bed height (cm) dividedby the linear flow rate (cm/h) applied during sample loading.2.2 Purification parametersGenerally,IgG from most species and Fc-fusion protein bind Protein G Agarose High Flowresin at neutral pH and physiological ionic strength, and are eluted at lowpH. The recommended buffers for purification listed below can be used as goodstarting conditions for your experiments: (1). Binding buffer: 20mM Sodiumphosphate, 150mM NaCl, pH7.2; 20mM Tris, 100mM NaCl, pH7-8; Phosphatebuffered saline (PBS), pH 7.4 (0.01M phosphate buffer, 0.0027M KCl, 0.14MNaCl). (2). Elution buffer: 100mMGlycine, pH 2.5-3.0.(3). Neutralize buffer: 1M Tris pH 8-9.2.3 Purification procedures1. Pack the column as described in "ColumnPacking" section. The recommended column height is within 5-20cm.2. Equilibrate the column at recommended flowrate with 5-10 column volumes of binding buffer to get a stable baseline.3. Calculate appropriate sample amount forloading. In principle, dynamic capacity is related to lots of parameters,such as antibody type, residence time, sample concentration, binding bufferand so on. Therefore, the maximum loading volume can be obtained by frontalanalysis for individual sample under specific binding conditions. Generally,the dynamic binding capacity is around 15-20mg/ml medium for 4-8min residencetime.4. Apply clarified sample of antibody ontocolumn. Samples need to be clarified by 0.45micron filter to remove anyparticles and colloids before application. It is recommended to dilutesamples of high protein concentration, such as anti-serum, with equal volumeof binding buffer to reduce sample viscosity.5. Wash column with 5 column volumes ofbinding buffer until UV level drop to baseline. Though not necessary for mostof the cases, optional intermediate washing step with salts or detergents mayhelp to remove impurities to some extent.6. Elute the column with 10 column volumes elution buffer.The most commonly used elution buffer is pH2.5 to 3.0; however, pH lower than2.5-3.0 is required for efficient elution of extremely strong bindingantibodies with high recovery. Non-ionic detergents, arginine and urea havebeen reported to improve antibody stability and avoid aggregation duringelution. Solvents such as 2-propanl can increase elution strength bydecreasing polarity of elution buffer.7. Neutralize the elution peak immediately with 1M Trisbuffer of pH 8.0-9.0.8. Re-equilibrate the column with 5-10 column volumes ofneutral binding buffer. |
| PURIFICATION CASES : | Sample: ClarifiedCHOSFM cellculture containing rat IgG mAb, 12mg/L titer.Binding buffer: PBS pH7.2Elution buffer: 100mMGlycine pH 2.7Column: Protein G AgaroseHigh Flow Resin packed into a 0.5x 5 (cm, i.d. x Height) 1ml columnLoad sample volume: 100ml, totally1.3mg human IgG1 mAb (Elisa).Flow rate: 1ml/min(300cm/hr) for loading and elutionUV280nm detection.Peak neutralizedimmediately with 1/20(v/v) 1M Tris pH8.0 after elution.Purity assay by 13% reducedgradient SDS-PAGE.110 mL SFM CHO cellculture with 12 mg/L titer was loaded onto Protein G Agarose High Flow Resinfor rat IgG mAb capture. And finally 1.3mg mAb (BCA Assay) with >95%purity was obtained successfully. |
| CLEAN IN PLACE(CIP) : | Clean in place(CIP)is the important procedures for removing very tightly bound, precipitated ordenatured proteins, DNA and Iipids, so as to maintain performance andcapacity of the column. Recommended CIP procedures for Protein G Agarose HighFlow Resin are as below:ClP procedures:(1). Wash thecolumn with 3 to 5 column volumes of binding buffer.(2). Backflush with1 to 2 column voIumes of 6 M Urea or 6M Gua-HCl withcontact time of 10 minutes.(3). Washimmediately with 5-10 column volumes of binding buffer at pH 7-8 to removeClP reagents.CIP isusually performed immediately after the elution. CIP reagents concentration,contact time and frequency are typically the main parameters to vary duringthe optimization of the CIP. The nature of the feed material will ultimatelydetermine the final CIP. However, the general recommendation is to clean thecolumn at least every 5 cycles during normal use. Depending on the nature ofthe contaminants, different protocols may have to be combined, for example0.1M acetate acid every cycle and 6M Gua-HCl every 5 cycles.Denaturantssuch as 8M Urea can remove the precipitated proteins, and non-ionic detergentssuch as 0.1% Triton X-100 or solvents such as 70% Ethanol can removehydrophobic substances. |
| SANITIZATION : | Sanitizationreduces microbial contamination of the chromatography column to a minimum.Protein G Agarose High Flow Resin allows the use of 0.1M acetate acid in 20%ethanol as sanitizing agent for sanitization.Sanitizationprocedures:1. Wash the columnwith 3 column volumes of binding buffer.2. Wash with0.1 M acetic acid in 20% ethanol for sanitization. Contact time of one houris recommended.3. Washimmediately with at least 5 column volumes of sterile and filtered bindingbuffer at pH 7-8. |
| TROUBLE SHOOTING : | High columnbackpressure during purification1. Disconnectthe column with system and make sure no tubings or connectors in the systemcaused the high system pressure; always use tubings and connectors of rightinner diameters.2. Removeflow restrictor from systems if possible.3. Calibratethe pressure sensor in your systems.4. Make sureall buffers and samples be filtered through 0.22 or 0.45 micron disc. membranefor clarification. For small volume sample, 10000g@10-20min centrifugation isan alternative solution5. Lowerflow rate when use buffers of high viscosity or working at cold temperature,especially during sample loading.6. Replacetop screen net of column adapter in case of clogging.7. Lower thecolumn bed height to 20 cm or less, too high beds will cause high pressure.8. Perform athorough CIP procedure to restore the initial back pressure if column bedclogs. Unpack the column and wash media batch wise.9. Increasethe CIP frequency and optimize the CIP regent formulations.10. Avoidfreeze the medium or column during storage.Poor binding or lowcapacity1. Check thebinding affinity of your antibodies of interest to the ligand.2. Make sure the pHvalues of binding buffer and sample are pH 7-8.3. Adjustthe binding buffer and sample conditions to promote binding: increase saltconcentration to 0.5-1M NaCl, increase pH to 8.5-9.0, etc.4. Check ifthere exist some interference substances in binding buffer or samples, suchas high concentration of chaotropic substances.5. Lowerflow rate to give a residence time of 4-8min for sample loading.6. Check thehistory of the medium about how it has been cleaned and stored.Inefficient elution1. Check the pHvalue and composition for elution buffer.2. Try elutionbuffer of lower pH, for example pH 2.3.3. Introduce0.1-1% non-ionic detergents for elution, such as Tween 80 and Triton X-100.4. Use somechaotropic substances of low concentration in elution buffer.5. Introducesome solvents to decrease the polarity.6. Try otheraffinity media or other technologiesLow purity1. Reducethe sample holding time, lower purification temperature and always useprotease inhibitors in samples and buffers to avoid degradation.2. Try touse as mild as possible elution conditions to avoid antibodies aggregation,and be sure to neutralize peak collected immediately after elution.3. Introduce anintermediate washing step before elution to remove any non-specific bindingimpurities, and some commonly used substances in washing buffer includes 1MNaCl, 0.5M Tetramethylammonium Chloride or detergents.4. Introducean intermediate washing step before elution to remove any non-specificbinding impurities, and some commonly used substances in washing bufferincludes 1M NaCl, 0.5M Tetramethylammonium Chloride or detergents.5. Use pHlinear 5-10 column volumes gradient (for example, phosphate and citrate toform pH 7.3 to 3.0 gradient ) instead of stepwise elution and pool fractionsof high purity.6.Alternative chromatography techniques need to be combined with affinitychromatography for higher purity with a multistep purification strategy, suchas size exclusion and ion-exchange, etc. |
| STORAGE : | Store unused mediain its container at a temperature of 2 to 8°C for long term storage. Ensurethat the container is closed and fully tightened. Equilibrate packed columnswith 5-10 column volumes of 20% ethanol to prevent microbial growth. Athoroughly CIP procedure is recommended before long term storage. Afterstorage, equilibrate with binding buffer and ready for use. Never freeze themedia in case of generation of fine particles to increase the back pressure. |
| Publications : |
|
| ◆ Recombinant Proteins | ||
| Protein G-2092S | Recombinant Streptococcus Protein G | +Inquiry |
| COP33 | Recombinant Streptococcal Protein G | +Inquiry |
| COP10 | Recombinant Protein G, His-tagged | +Inquiry |
| Protein G-2093S | Recombinant Streptococcus Protein G(Type 4) | +Inquiry |
| Protein G-525 | Recombinant Group G streptococci Protein G Protein | +Inquiry |
Case 1: Kallaste A, et al. Infect Dis (Lond). 2022
Understanding the longevity of antibodies against SARS-CoV-2 infection is of utmost importance in predicting the further course of the pandemic and to plan vaccination strategies. Researchers conducted a longitudinal study of 123 COVID-19 patients and 45 SARS CoV-2 negative outpatients with upper respiratory tract infection. They analyzed the demographic and clinical features of the patients with COVID-19 in relation to different disease severities according to the WHO classification by performing LIPS with Protein G Sepharose beads to capture antibodies. The results showed that amongst the enrolled COVID-19 patients, 15 (12%) had mild, 42 (34%) had moderate, 39 (32%) had severe and 27 (22%) had critical disease courses; 79% of the patients were hospitalized. During follow-up, all patients had anti-SARS RBD-IgG levels above the cut-off value on all visits, but the antibody levels varied significantly between the different disease severity groups.
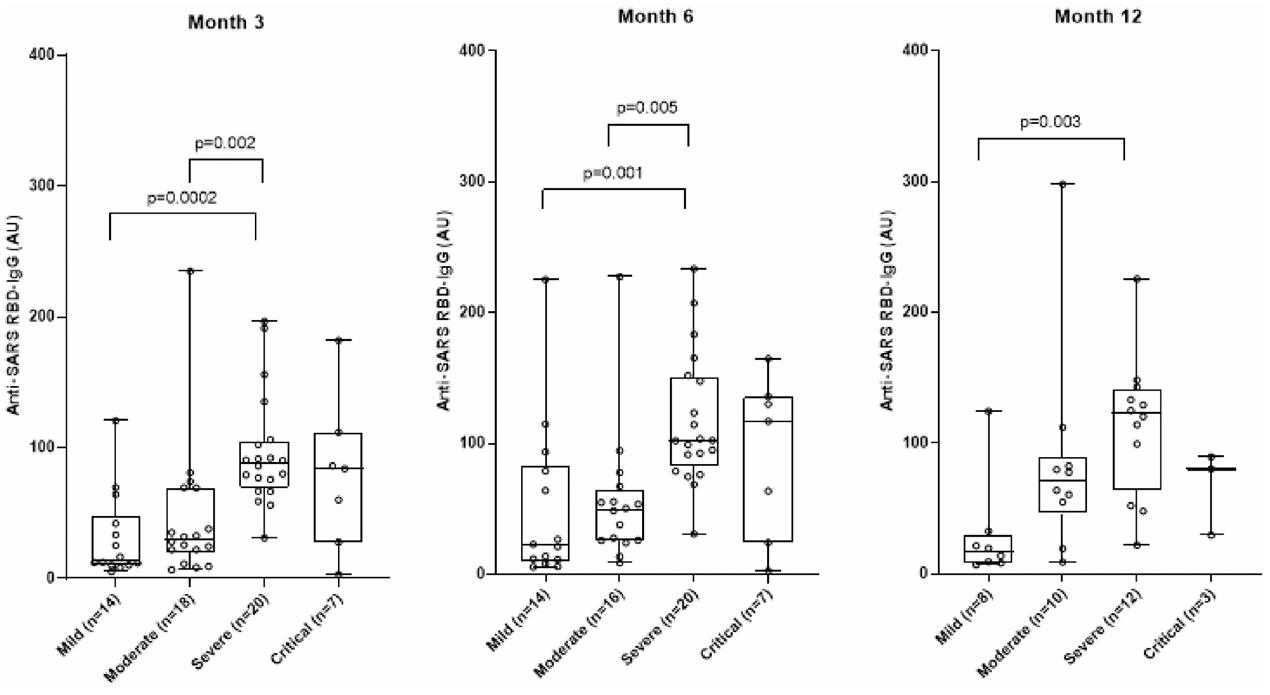
Fig1. Anti-SARS RBD-IgG in different COVID-19 severity groups during follow-up visits.
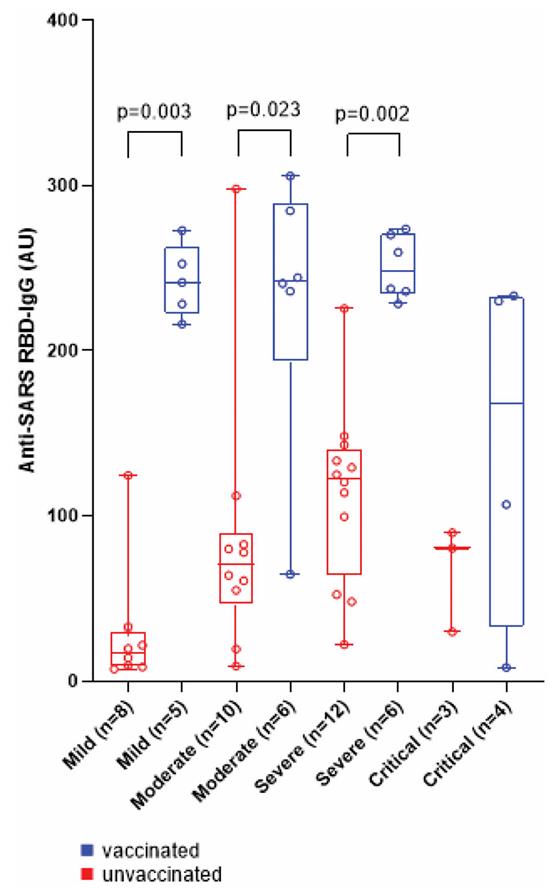
Fig2. Anti-SARS RBD-IgG in SARS-CoV-2 vaccinated (in blue) and unvaccinated (in red) patients with different COVID-19 severity groups at month 12.
Case 2: Haljasmägi L, et al. Sci Rep. 2020
SARS-CoV-2 infection has a risk to develop into life-threatening COVID-19 disease. Whereas age, hypertension, and chronic inflammatory conditions are risk factors, underlying host factors and markers for disease severity, e.g. requiring intensive care unit (ICU) treatment, remain poorly defined. To this end, researchers longitudinally profiled blood inflammation markers, antibodies, and 101 plasma proteins of hospitalized COVID-19 patients who did or did not require ICU admission. In LIPS assays, Protein G Sepharose beads were used to incubate HEK293 cell lysates. While essentially all patients displayed SARS-CoV-2-specific antibodies and virus-neutralization capacity within 12-15 days, a rapid, mostly transient upregulation of selective inflammatory markers including IL-6, CXCL10, CXCL11, IFNγ, IL-10, and monocyte-attracting CCL2, CCL7 and CCL8, was particularly evident in ICU patients. In addition, there was consistent and sustained upregulation of apoptosis-associated proteins CASP8, TNFSF14, HGF, and TGFB1, with HGF discriminating between ICU and non-ICU cohorts.
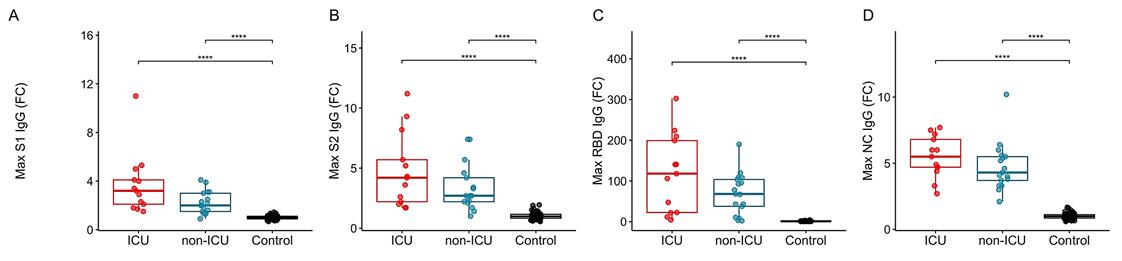
Fig1. Peak levels of IgG antibodies against SARS-CoV-2 spike protein subunits (A) S1, (B) S2, (C) its receptor binding domain (RBD) and (D) nucleocapsid protein (NC).
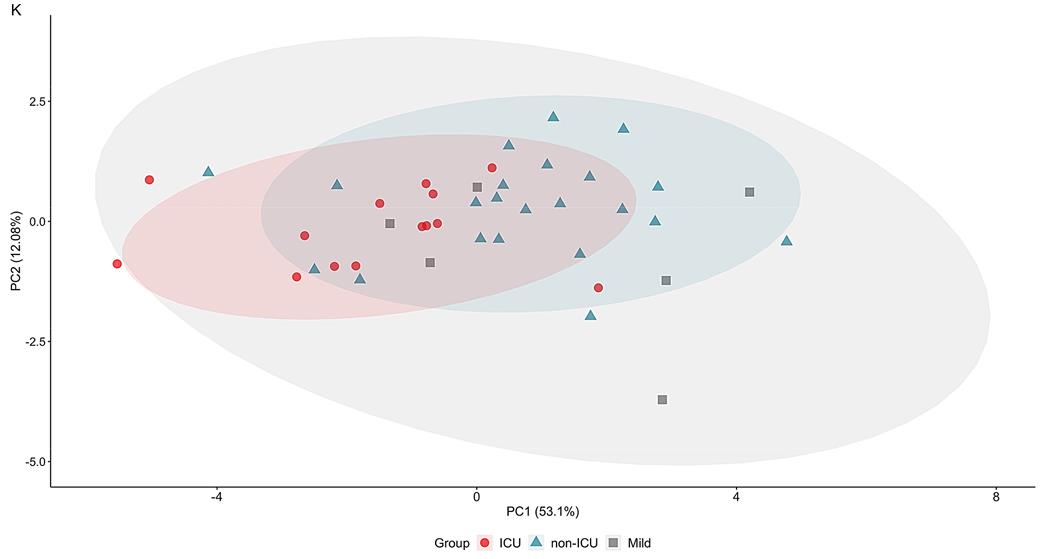
Fig2. PCA plot calculated based on 10 markers' average levels consistently upregulated in COVID-19.
Protein G agarose is a widely used affinity resin in protein purification and immunoprecipitation applications. Some common applications of Protein G agarose include:
Antibody purification: Protein G agarose is used to purify antibodies from serum, ascites fluid, or hybridoma cell culture supernatants. It binds to the Fc region of various classes of immunoglobulins, allowing for efficient and specific antibody purification.
Immunoprecipitation (IP): Protein G agarose is commonly used in immunoprecipitation assays to capture and isolate a specific protein or protein complex along with its associated antibodies from a biological sample.
Co-immunoprecipitation (Co-IP): Protein G agarose can be used to facilitate the co-IP of protein complexes by capturing a target protein of interest along with its interacting proteins.
Chromatin immunoprecipitation (ChIP): Protein G agarose is widely used in chromatin immunoprecipitation assays to isolate protein-DNA complexes and study protein-DNA interactions.
Protein-protein interaction studies: Protein G agarose can be used to study protein-protein interactions by capturing one protein of interest along with its interacting partners in a protein complex.
Immunoaffinity chromatography: Protein G agarose is utilized in immunoaffinity chromatography to purify and isolate specific antigens or proteins in a sample based on their interaction with antibodies.
Biomolecular interaction studies: Protein G agarose is employed in studies involving biomolecular interactions, such as protein-ligand binding assays, protein-nucleic acid interactions, and protein-protein binding studies.
Overall, Protein G agarose is a versatile tool in the field of protein purification and characterization, immunology, and molecular biology, and its applications extend to various research areas that require the specific capture and isolation of target proteins and protein complexes.
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All Protein G Products
Required fields are marked with *
My Review for All Protein G Products
Required fields are marked with *



