Recombinant Human X-linked Inhibitor Of Apoptosis, GST-tagged
| Cat.No. : | XIAP-1503H |
| Product Overview : | Recombinant full-length human XIAP was expressed by baculovirus inSf9insect cells using a C-terminal GST tag. MW = 84 kDa. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | Sf9 Cells |
| Tag : | GST |
| Description : | XIAP is a member of a family of proteins which inhibit apoptosis and act as mammalian cell-death suppressors. XIAP inhibits at least 2 members of the caspase family of cell-death proteases, caspase-3 and caspase-7. In addition, XIAP inhibits apoptosis induced by menadione, a potent inducer of free radicals, and ICE. Furthermore, XIAP can mediate protection of cells from apoptosis by utilizing both a JNK1 activation pathway that involves ILPIP and a caspase inhibition pathway that is independent of ILPIP. Disruption of the XIAP gene in human colon cancer cells can enhance sensitivity of these cells to exogenously added TRAIL suggesting that XIAP is a nonredundant modulator of TRAIL-mediated apoptosis. |
| Sequence : | Full-length. |
| Applications : | Western Blot. |
| Storage And Stability : | Store product at -70℃. For optimal storage, aliquot target into smaller quantities after centrifugation and store at recommended temperature. For most favorable performance, avoid repeated handling and multiple freeze/thaw cycles. |
| Gene Name | XIAP X-linked inhibitor of apoptosis [ Homo sapiens ] |
| Synonyms | XIAP; X-linked inhibitor of apoptosis; API3; ILP1; MIHA; XLP2; BIRC4; baculoviral IAP repeat-containing protein 4; OTTHUMP00000196392; apoptosis inhibitor 3; baculoviral IAP repeat-containing 4; E3 ubiquitin-protein ligase XIAP; Inhibitor of apoptosis protein 3; X-linked inhibitor of apoptosis protein; X-linked IAP; IAP-like protein; HILP; IAP3; EC 6.3.2.-; OTTHUMP00000023975; hILP |
| Gene ID | 331 |
| mRNA Refseq | NM_001167 |
| Protein Refseq | NP_001158 |
| MIM | 300079 |
| UniProt ID | P98170 |
| Chromosome Location | Xq25 |
| Pathway | Apoptosis; Focal adhesion; NOD-like receptor signaling pathway; Pathways in cancer; Small cell lung cancer; Ubiquitin mediated proteolysis |
| Function | caspase inhibitor activity; ligase activity; metal ion binding; peptidase inhibitor activity; protein binding; zinc ion binding |
| ◆ Recombinant Proteins | ||
| Xiap-626M | Recombinant Mouse Xiap Protein, MYC/DDK-tagged | +Inquiry |
| XIAP-1503H | Recombinant Human X-linked Inhibitor Of Apoptosis, GST-tagged | +Inquiry |
| XIAP-0711H | Recombinant Human XIAP Protein (Y120-P260), Tag Free | +Inquiry |
| XIAP-246H | Recombinant Human XIAP Protein, His/StrepII-tagged | +Inquiry |
| XIAP-247H | Recombinant Human XIAP protein, His-SUMO-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| XIAP-263HCL | Recombinant Human XIAP 293 Cell Lysate | +Inquiry |
Mutual regulation between OGT and XIAP to control colon cancer cell growth and invasion
Journal: Cell Death & Disease PubMed ID: 32994395 Data: 2020/9/29
Authors: Hyeon Gyu Seo, Han Byeol Kim, Jin Won Cho
Article Snippet:Recombinant His-Ubiquitin protein and His-UCHL5 were purchased from R&D systems (Minneapolis, MN, USA) and LSBio (Seattle, WA, USA), respectively.Recombinant His-Ubiquitin protein and His-UCHL5 were purchased from R&D systems (Minneapolis, MN, USA) and LSBio (Seattle, WA, USA), respectively.. Recombinant GST-XIAP was purchased from Creative BioMart (Shirley, NY, USA).. Glutathione agarose and Ni-NTA agarose were purchased from Qiagen (Hilden, Germany).Glutathione agarose and Ni-NTA agarose were purchased from Qiagen (Hilden, Germany).
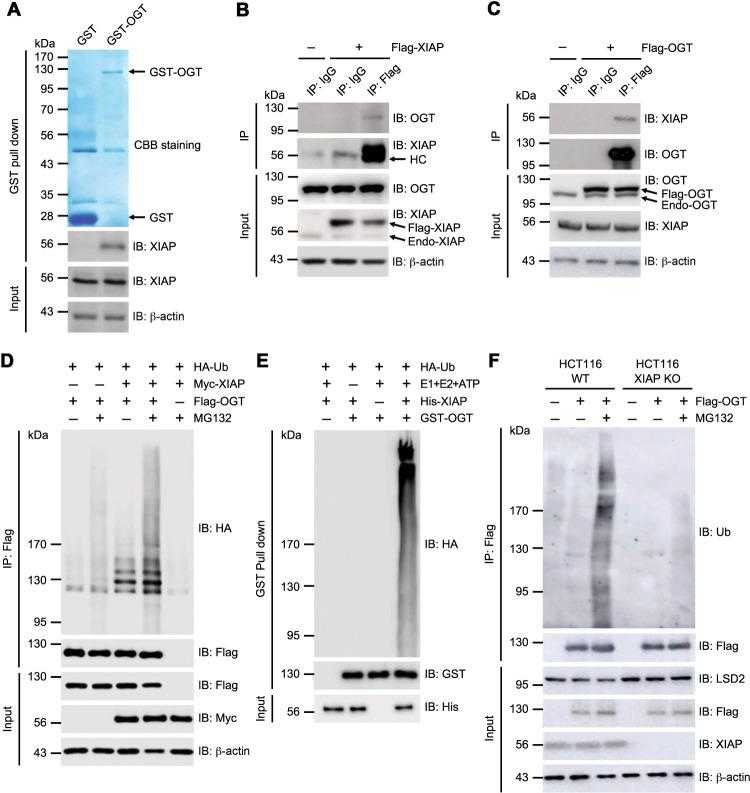
a HCT16 cell lysates were incubated with immobilized recombinant
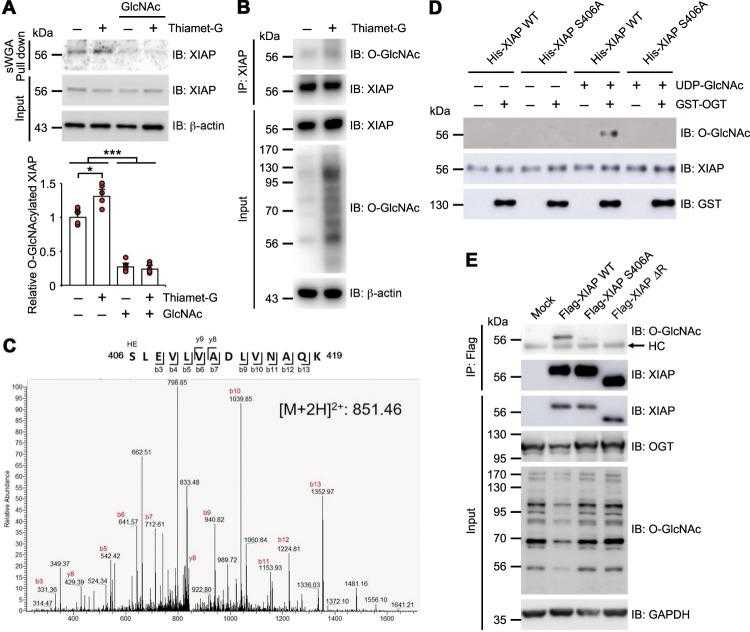
a HCT116 cells were either treated with 1 μM of Thiamet-G for 4 h or not treated with any Thiamet-G. Cell lysates were then subjected to sWGA lectin affinity purification and analyzed with Western blotting for the presence of endogenous
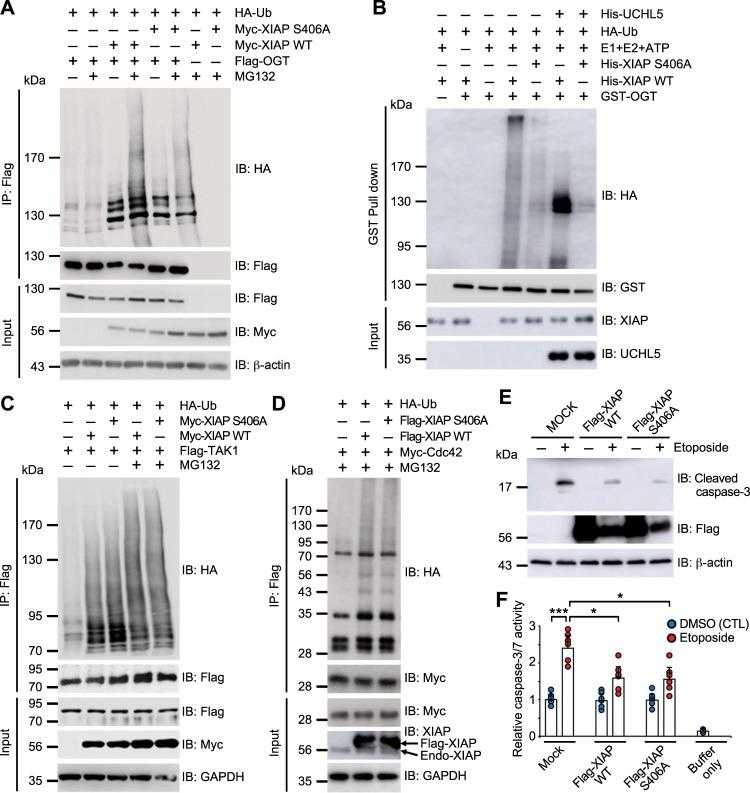
a Expression vectors encoding Flag-OGT and HA-Ub were transfected into HCT116 cells transiently overexpressing
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All XIAP Products
Required fields are marked with *
My Review for All XIAP Products
Required fields are marked with *



