ALS Related Molecules
Related Symbol Search List
- AOX1
- CHMP2B
- CSF3R
- DCTN1
- DCTN2
- GDNF
- IGF1R
- RET
- SLC1A2
- SLC1A3
- SNCA
- SNCB
- SNCG
- SOD1
- SOD2
- TARDBP
- UBE2K
- UCP1
- VAPB
- VEGFA
Immunology Background
About ALS and Related Molecules
ALS, which stands for amyotrophic lateral sclerosis, is a progressive neurodegenerative disorder that primarily affects motor neurons in the brain and spinal cord. It results in the progressive loss of voluntary muscle control and ultimately leads to paralysis. While the exact cause of ALS remains elusive, extensive research has identified several molecules and genetic factors that are associated with the disease, such as superoxide dismutase 1 (SOD1), TAR DNA-binding protein 43 (TDP-43), and C9orf72. These molecules play crucial roles in various cellular processes and pathways, and their dysregulation or dysfunction contributes to the development and progression of ALS.
Other molecules and pathways involved in ALS include protein aggregation pathways, oxidative stress, glutamate excitotoxicity, neuroinflammation, and mitochondrial dysfunction. These processes contribute to the progressive degeneration of motor neurons observed in ALS.
Understanding the role of these molecules and pathways in ALS is crucial for the development of potential therapeutic strategies. Targeting these molecules and pathways may help to alleviate the symptoms of ALS and slow down disease progression. However, much research is still needed to unravel the complexity of ALS and develop effective treatments.
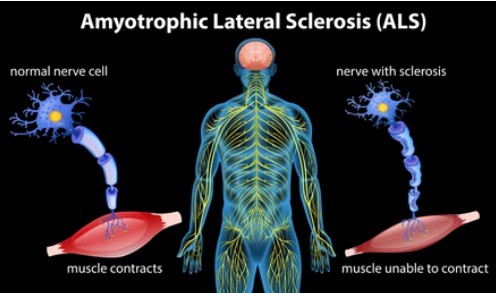
Biological Functions of ALS-Related Molecules
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that primarily affects motor neurons in the brain and spinal cord. Several molecules have been implicated in the pathogenesis of ALS. Here are the biological functions of some ALS-related molecules:
Superoxide dismutase 1 (SOD1): Mutations in the SOD1 gene are associated with a subset of familial ALS cases. SOD1 is an enzyme that plays a critical role in cellular antioxidant defense. It catalyzes the conversion of superoxide radicals into oxygen and hydrogen peroxide. However, mutant SOD1 can acquire toxic properties, leading to oxidative stress, mitochondrial dysfunction, and neurodegeneration.
TAR DNA-binding protein 43 (TDP-43): TDP-43 is an RNA-binding protein that is involved in various aspects of RNA metabolism, including transcription, RNA splicing, and transport. In ALS, abnormal accumulation of TDP-43 in the cytoplasm of affected motor neurons is a pathological hallmark. Dysregulation of TDP-43 function can disrupt normal RNA processing and lead to neurotoxicity and neuronal loss.
C9orf72: The hexanucleotide repeat expansion in the C9orf72 gene is the most common genetic cause of both familial and sporadic ALS. The exact biological function of C9orf72 is not fully understood, but it is thought to play a role in membrane trafficking and autophagy. Dysfunction of C9orf72 due to the repeat expansion leads to impaired cellular processes, including abnormal protein aggregation and impaired clearance mechanisms.
FUS (Fused in Sarcoma): FUS is another RNA-binding protein involved in various cellular processes, including transcription, RNA splicing, and transport. Mutations in the FUS gene have been associated with familial ALS. Similar to TDP-43, abnormal accumulation of FUS in the cytoplasm of motor neurons is observed in ALS. Dysregulated FUS function can disrupt RNA metabolism and contribute to neurodegeneration.
Ubiquilin 2 (UBQLN2): Mutations in the UBQLN2 gene have been identified in both familial and sporadic ALS cases. UBQLN2 is involved in protein degradation pathways, particularly the ubiquitin-proteasome system and autophagy. Dysregulation of UBQLN2 function may lead to impaired protein clearance, accumulation of protein aggregates, and neuronal dysfunction in ALS.
VCP (Valosin-containing protein): Mutations in the VCP gene are associated with a rare form of ALS called ALS with inclusion body myopathy (ALS-IBM). VCP is an ATPase enzyme that plays a role in protein degradation processes, including the ubiquitin-proteasome system and autophagy. Mutant VCP can disrupt protein homeostasis and impair the clearance of misfolded proteins, contributing to neurodegeneration.
These molecules and their associated pathways are involved in various cellular processes, including protein homeostasis, RNA metabolism, oxidative stress response, and proteostasis. Dysregulation or dysfunction of these molecules in ALS can lead to the accumulation of protein aggregates, impaired cellular processes, and ultimately motor neuron degeneration. Understanding the biological functions of ALS-related molecules is crucial for unraveling the mechanisms underlying the disease and developing potential therapeutic strategies.
Significance and Application Areas of ALS-related Molecular Research
ALS-related molecular research holds significant importance and has several application areas that contribute to our understanding of the disease and potential therapeutic interventions. Some of the key significance and application areas of ALS-related molecular research include:
Disease Mechanisms: Studying ALS-related molecules allows researchers to unravel the underlying mechanisms of the disease. By understanding how these molecules function, how they interact with each other, and how their dysregulation contributes to motor neuron degeneration, researchers can gain insights into the cellular and molecular processes involved in ALS pathogenesis.
Biomarkers: Identification of ALS-related molecules and their alterations can help in the discovery and validation of biomarkers. Biomarkers are measurable indicators that can provide information about the presence, severity, progression, or response to treatment of a disease. ALS biomarkers can aid in early diagnosis, monitoring disease progression, and evaluating treatment efficacy.
Therapeutic Targets: ALS-related molecules can serve as potential therapeutic targets for the development of treatments. By understanding the roles of these molecules in disease progression, researchers can design interventions aimed at modulating their activity or expression to mitigate motor neuron degeneration and slow down disease progression. This knowledge can guide the development of novel therapeutic strategies, including small-molecule drugs, gene therapies, and other innovative approaches.
Drug Discovery and Development: ALS-related molecular research provides a foundation for drug discovery and development efforts. By identifying key molecular targets and pathways involved in ALS, researchers can screen and develop compounds that specifically target these molecules or modulate their activity. This can lead to the development of new drugs or repurposing of existing drugs for ALS treatment.
Animal Models and Drug Testing: ALS-related molecular research informs the development and utilization of animal models that mimic aspects of the disease. These models allow researchers to study the effects of manipulating specific molecules or pathways, test potential therapeutics, and gain insights into disease mechanisms in a controlled setting before translating findings to human clinical trials.
Overall, ALS-related molecular research has the potential to advance our understanding of the disease, identify diagnostic and prognostic markers, discover novel therapeutic targets, facilitate drug discovery and development, enable personalized medicine approaches, and contribute to the development of effective treatments for ALS patients.
Available Resources for ALS-Related Molecules
Creative BioMart offers a broad range of products and services to support research into ALS disease and related molecules. Our offerings include recombinant proteins, cell and tissue lysates, and protein pre-coupled beads. We also offer custom services to meet specific research needs. Additionally, we provide resource support, including pathways, protein functions, interacting proteins, and relevant articles, to enhance understanding and study of these molecules. Explore the ALS-related molecules below for more comprehensive resources.
We are dedicated to providing you with high-quality research tools and services to help you achieve successful scientific outcomes. If you have any further questions or require custom services, please feel free to contact us at any time.
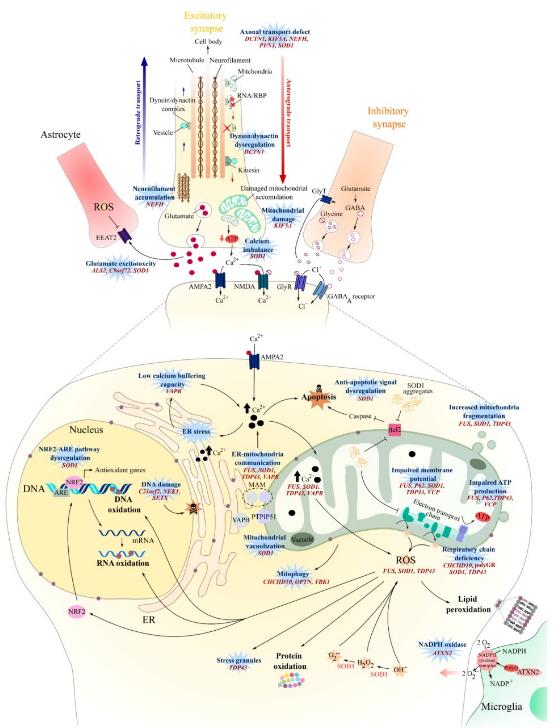 Fig.2 Oxidative stress, mitochondrial dysfunction, axonal transport, and glutamate excitotoxicity in amyotrophic lateral sclerosis (ALS). (Le Gall, et al., 2020)
Fig.2 Oxidative stress, mitochondrial dysfunction, axonal transport, and glutamate excitotoxicity in amyotrophic lateral sclerosis (ALS). (Le Gall, et al., 2020)
References:
- Feldman, Eva L., et al. "Amyotrophic lateral sclerosis." The Lancet 400.10360 (2022): 1363-1380.
- Le Gall, Laura, et al. "Molecular and cellular mechanisms affected in ALS." Journal of personalized medicine 10.3 (2020): 101.
- Yang, Xiaoming, et al. "Amyotrophic lateral sclerosis: Molecular mechanisms, biomarkers, and therapeutic strategies." Antioxidants 10.7 (2021): 1012.

