Recombinant Human SMURF2, GST-tagged
| Cat.No. : | SMURF2-138H |
| Product Overview : | Recombinant human SMURF2 (amino acid residues 1-748), with N-terminal GST, was expressed in E.coli. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | GST |
| Protein Length : | 1-748 a.a. |
| Description : | The enzymes of the ubiquitylation pathway play a pivotal role in a number of cellular processes including the regulated and targeted proteasome-dependent degradation of substrate proteins. Three classes of enzymes are involved in the process of ubiquitylation; activating enzymes (E1s), conjugating enzymes (E2s) and protein ligases (E3s). Smad-Specific E3 Ubiquitin Protein Ligase 1 (SMURF2) is a member of the E3 protein ligase family and cloning of the human gene was first described by Kavsak et al. (2000). SMURF2 is a HECT domain ubiquitin E3 ligase that has been shown to regulate cell polarity, senescence and tumor suppression (Blank et al., 2012). |
| Form : | 50 mM HEPES pH 7.5, 150 mM sodium chloride, 2 mM dithiothreitol, 10% glycerol |
| Molecular Mass : | ~114kDa |
| Storage : | 12 months at -70°C. Avoid multiple freeze/thaw cycles. |
| Concentration : | 0.5mg/ml |
| Gene Name | SMURF2 SMAD specific E3 ubiquitin protein ligase 2 [ Homo sapiens ] |
| Official Symbol | SMURF2 |
| Synonyms | SMURF2; SMAD specific E3 ubiquitin protein ligase 2; E3 ubiquitin-protein ligase SMURF2; hSMURF2; E3 ubiquitin ligase SMURF2; SMAD ubiquitination regulatory factor 2; SMAD-specific E3 ubiquitin-protein ligase 2; MGC138150; DKFZp686F0270; |
| Gene ID | 64750 |
| mRNA Refseq | NM_022739 |
| Protein Refseq | NP_073576 |
| MIM | 605532 |
| UniProt ID | Q9HAU4 |
| Chromosome Location | 17q22-q23 |
| Pathway | Adaptive Immune System, organism-specific biosystem; Antigen processing: Ubiquitination and Proteasome degradation, organism-specific biosystem; BMP receptor signaling, organism-specific biosystem; Class I MHC mediated antigen processing & presentation, organism-specific biosystem; Endocytosis, organism-specific biosystem; |
| Function | SMAD binding; acid-amino acid ligase activity; identical protein binding; ligase activity; protein binding; ubiquitin-protein ligase activity; |
| ◆ Recombinant Proteins | ||
| SMURF2-1714H | Recombinant Human SMURF2 Protein (1-748 aa), His-tagged | +Inquiry |
| SMURF2-980HFL | Recombinant Full Length Human SMURF2 Protein, C-Flag-tagged | +Inquiry |
| SMURF2-5744H | Recombinant Human SMURF2 protein, His-tagged | +Inquiry |
| SMURF2-4835H | Recombinant Human SMURF2 protein, His-tagged | +Inquiry |
| SMURF2-0440H | Recombinant Human SMURF2 Protein (S2-E748), His tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| SMURF2-1646HCL | Recombinant Human SMURF2 293 Cell Lysate | +Inquiry |
KRAS Protein Stability Is Regulated through SMURF2: UBCH5 Complex-Mediated ?-TrCP1 Degradation
Journal: Neoplasia (New York, N.Y.) PubMed ID: 24709419 Data: 2014/2/1
Authors: Shirish Shukla, Uday SankarAllam, Dipankar Ray
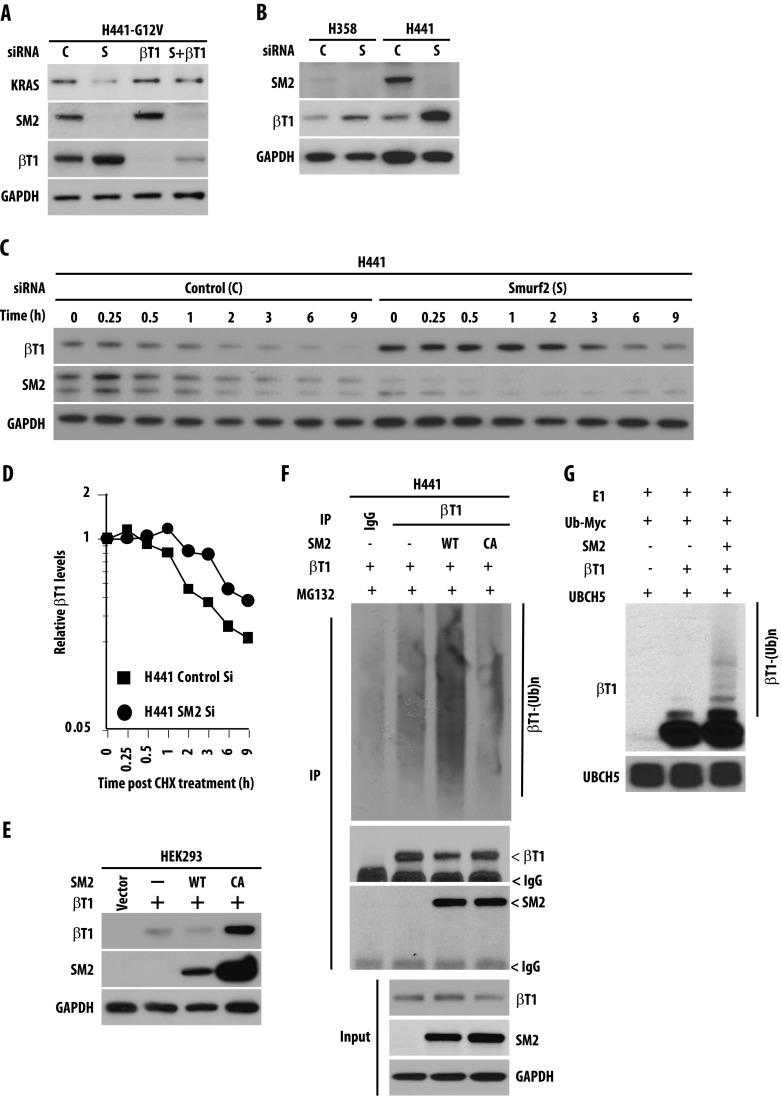
Article Snippet:The in vitro ubiquitination reaction was carried out in a 15-μl reaction volume containing reaction buffer [250 mM Tris-HCl (pH 7.5), 50 mM MgCl 2 , 50 μM DTT, and 20 mM ATP), 10 βg of Myc-tagged ubiquitin (Cat. No. U-115), 0.35 βg of UBE1 (Cat. No. E305), and 0.5 βg of UBCH5 (Cat. No. E2-616; all from Boston Biochemicals), and Flag-tagged β-TrCP1 was overexpressed in HEK293 cells and pulled down using affi-Flag (M2) beads.(pH 7.5), 50 mM MgCl 2 , 50 μM DTT, and 20 mM ATP), 10 βg of Myc-tagged ubiquitin (Cat. No. U-115), 0.35 βg of UBE1 (Cat. No. E305), and 0.5 βg of UBCH5 (Cat. No. E2-616; all from Boston Biochemicals), and Flag-tagged β-TrCP1 was overexpressed in HEK293 cells and pulled down using affi-Flag (M2) beads. ... Human recombinant SMURF2 protein (Cat. 468H; Creative BioMart, New York, NY) was then added, and the reaction mixtures were incubated at 37°C for 2 hours.. The reaction was terminated after boiling with 4x gel loading dye.The reaction was terminated after boiling with 4x gel loading dye.
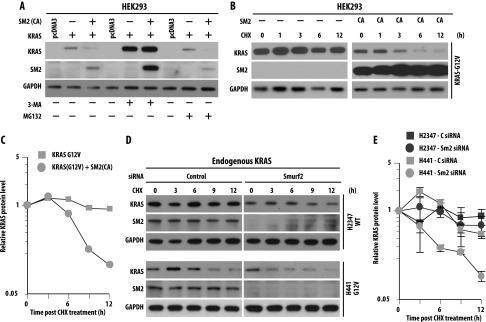
![SMURF2 ubiquitin ligase activity controls mutant KRAS steady-state levels. (A) H358 (KRASG12C), H441 (KRASG12V), and H2347 (KRASWT) human lung adenocarcinoma cells were transfected with either control (C) or Smurf2 (S) siRNA. Forty-eight hours posttransfection, cell lysates were subjected to immunoblot analysis using specified antibodies. (B) Quantification of KRAS steady-state level in H358, H441, and H2347 cells on C or S siRNA. Immunoblot obtained from A were scanned and quantified using ImageJ software, and KRAS steady-state levels were normalized using GAPDH as loading control. Relative KRAS protein levels were obtained, and mean ± SEM values were calculated from three independent experiments; * denotes significant difference from control at P < .05; **, denotes significant difference from control at P < .005; NS, not significant. (C) H441 cells were transfected with Smurf2 si-R (SM2-siR) construct before siRNA-mediated Smurf2 knockdown. Cell lysates were prepared 24 hours post siRNA transfection and immunoblotted using indicated antibodies. (D) HEK293 cells were co-transfected with Myc-tagged KRAS [either wild type or various mutants (G12D, V, C, or S)] in the presence or absence of FLAG-tagged SMURF2 [either wild type or C716A (CA) mutants]. Twelve hours post-transfection, cell lysates were prepared and subjected to immunoblot analysis using indicated antibodies.](productimages/extendimages/pmc03978392__neo1602_0115_fig001.jpg)
Ubiquitin ligase activity of
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All SMURF2 Products
Required fields are marked with *
My Review for All SMURF2 Products
Required fields are marked with *



