Recombinant Human SMPD1, His tagged
| Cat.No. : | SMPD1-656H |
| Product Overview : | Recombinant Human SMPD1 full length (NP_000534.3) (Met 1-Cys 631), fused with a polyhistidine tag at the C-terminus, was produced in Baculovirus-Insect cells. |
| Availability | January 27, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | Insect Cells |
| Tag : | His |
| Protein Length : | 1-631 a.a. |
| Form : | Lyophilized from sterile 50mM Tris, 100mM NaCl, pH 8.0, 0.1% OGP, 10% glycerol |
| Molecular Mass : | The secreted recombinant human SMPD1 consists of 518 amino acids and predicts a molecular mass of 65 kDa as estimated by SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 eu per μg of the protein as determined by the LAL method. |
| Stability : | Samples are stable for up to twelve months from date of receipt at -70oC. |
| Storage : | Store it under sterile conditions at -20oC~-70oC. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution. Centrifuge the vial at 4℃ before opening to recover the entire contents. |
| Full Length : | Full L. |
| Publications : |
| Gene Name | SMPD1 sphingomyelin phosphodiesterase 1, acid lysosomal [ Homo sapiens ] |
| Official Symbol | SMPD1 |
| Gene ID | 6609 |
| mRNA Refseq | NM_000543 |
| Protein Refseq | NP_000534 |
| MIM | 607608 |
| UniProt ID | P17405 |
| ◆ Recombinant Proteins | ||
| SMPD1-6318H | Recombinant Human SMPD1 Protein (Gly319-Gly579), N-His tagged | +Inquiry |
| SMPD1-1242M | Recombinant Mouse SMPD1 protein, His-GST-tagged | +Inquiry |
| SMPD1-6851H | Recombinant Human SMPD1 protein, His & T7-tagged | +Inquiry |
| Smpd1-2552M | Active Recombinant Mouse Smpd1 protein(Met1-Leu626), His-tagged | +Inquiry |
| SMPD1-7354H | Recombinant Human SMPD1 protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| SMPD1-716HCL | Recombinant Human SMPD1 cell lysate | +Inquiry |
| SMPD1-486MCL | Recombinant Mouse SMPD1 cell lysate | +Inquiry |
| SMPD1-702HCL | Recombinant Human SMPD1 cell lysate | +Inquiry |
Essential role for acid sphingomyelinase-inhibited autophagy in melanoma response to cisplatin
Journal: Oncotarget PubMed ID: 27107419 Data: 2016/5/3
Authors: Davide Cervia, Emma Assi, Cristiana Perrotta
Article Snippet:Cisplatin (Cisplatino Teva) was from Teva Pharma Italia (Milano, Italy).Cisplatin (Cisplatino Teva) was from Teva Pharma Italia (Milano, Italy).. Human A-SMase (Recombinant Human SMPD1, His-tagged) was purchased from Creative BioMart (Shirley, NY, USA).. Annexin V-Fluorescein Isothiocianate (FITC) and PI were obtained from Life Technologies (Monza, Italy) and eBioscience (San Diego, CA, USA), respectively.Annexin V-Fluorescein Isothiocianate (FITC) and PI were obtained from Life Technologies (Monza, Italy) and eBioscience (San Diego, CA, USA), respectively.
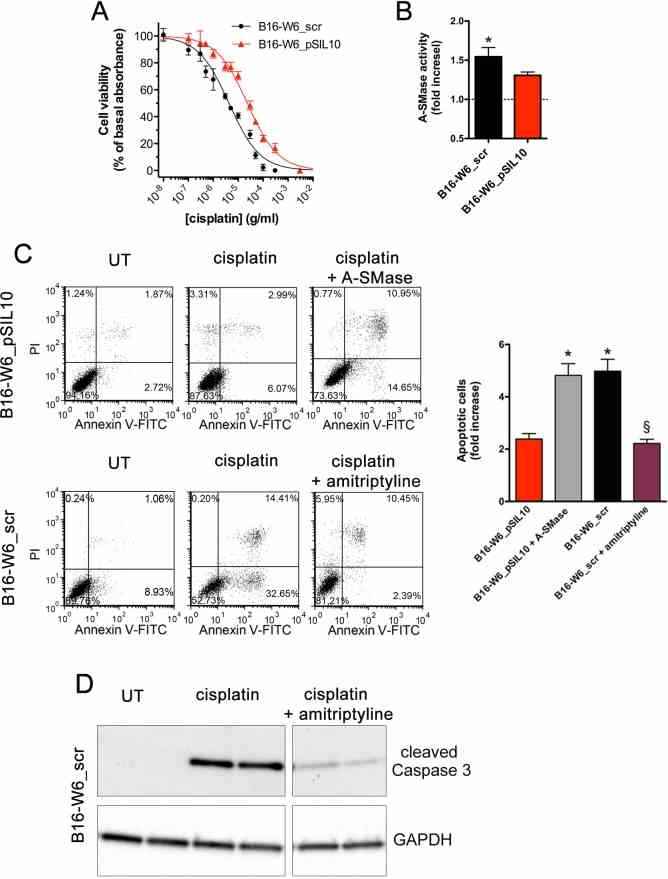
A. Dose-response curves of the effects of cisplatin on the viability of B16-W6_scr and B1-W6_pSIL10, as measured by the MTT assay ( n = 10). B. Measurement of
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All SMPD1 Products
Required fields are marked with *
My Review for All SMPD1 Products
Required fields are marked with *



