Producing Recombinant Proteins with the Baculovirus-Insect Cell System
Development History of BEVS: From Pest Control to Protein Powerhouse
Our journey begins not in a high-tech lab, but in agriculture. Baculoviruses, primarily infecting insects like moths and butterflies, were initially studied for their potential as biological pesticides. A key breakthrough came in the early 1980s. Researchers like Max Summers and Gale Smith made a pivotal discovery: they realized that a specific region of the baculovirus genome, the polyhedrin gene, was non-essential for virus replication in cell culture. Polyhedrin produces massive amounts of protein to form protective occlusion bodies in nature – essentially making it a natural, high-yield promoter. They ingeniously replaced this polyhedrin gene with foreign genes, demonstrating that insect cells infected with these modified viruses could produce large quantities of the foreign protein. This was the birth of BEVS as we know it. Since then, continuous refinements in vectors (like the development of faster dual-expression systems such as Bac-to-Bac and flash BAC), cell lines, and techniques have cemented BEVS as a gold standard for producing complex eukaryotic proteins.
Let's see the timeline of the BEVS development history
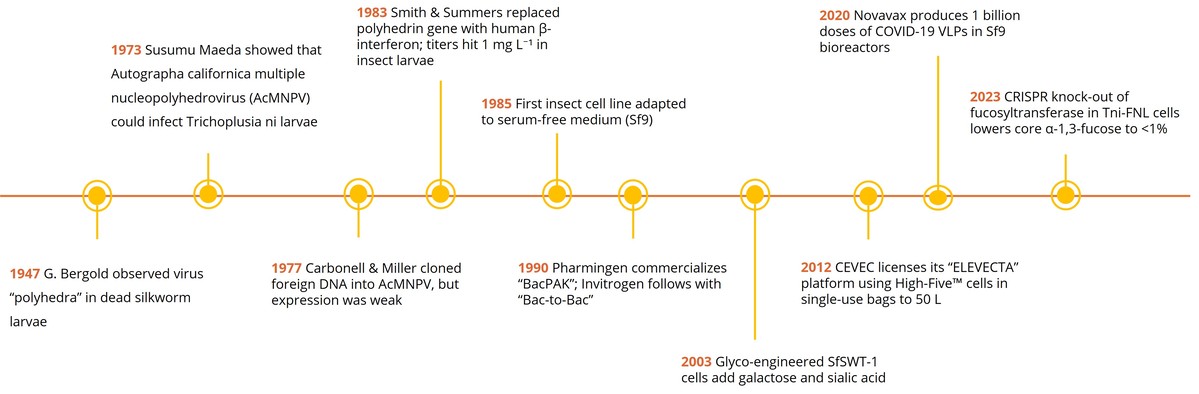 Fig1. BEVS development timeline
Fig1. BEVS development timeline1947 – Bergold observed virus "polyhedra" in dead silkworm larvae.
1973 – Susumu Maeda showed that Autographa californica multiple nucleopolyhedrovirus could infect Trichoplusia ni larvae.
1977 – Carbonell & Miller cloned foreign DNA into AcMNPV, but expression was weak.
1983 – Smith & Summers replaced the polyhedrin gene with human β-interferon; titers hit 1 mg per Liter in insect larvae.
1985 – First insect cell line (Sf9) adapted to serum-free medium.
1990 – Pharmingen commercializes "BacPAK"; Invitrogen follows with "Bac-to-Bac".
2003 – Glyco-engineered SfSWT1 cells add galactose and sialic acid.
2012 – CEVEC licenses its "ELEVECTA" platform using High-Five cells in single-use bags to 50 Liter.
2020 – Novavax produces 1 billion doses of COVID19 VLPs in Sf9 bioreactors.
2023 – CRISPR knock-out of fucosyltransferase in Tni-FNL cells lowers core α-1,3-fucose to <1 %.
What is a Baculovirus?
Baculoviruses, which belong to the family Baculoviridae, are a group of DNA viruses that infect insects. These viruses are very small, rod-shaped structures, composing of a protein coat containing a small amount of DNA which encodes components for further viral reproduction.
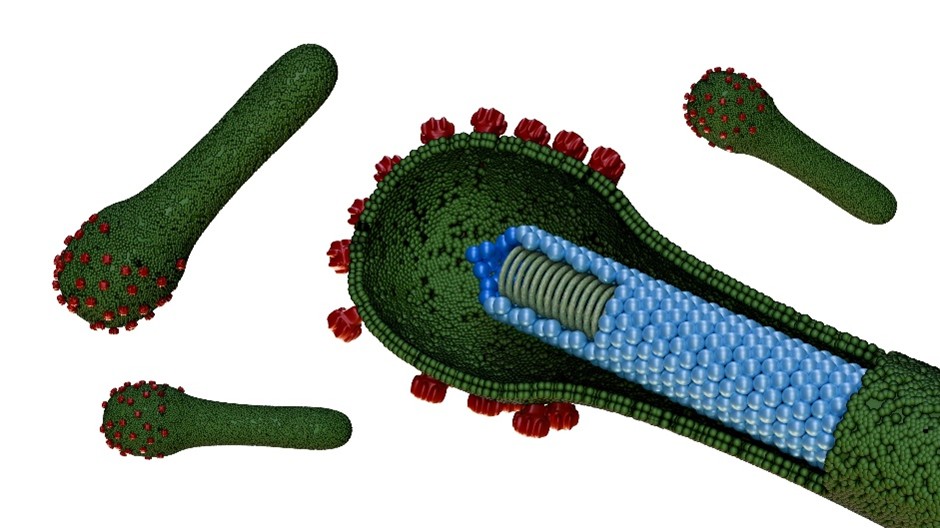 Fig2. Baculoviruses
Fig2. BaculovirusesBaculoviruses exist in two virion phenotypes. Budded virus carries a single envelope and uses the GP64 (or its homolog fusolin in some groups) to drive efficient cell-to-cell spread within the insect host. In contrast, occlusion-derived virus is embedded in a protective polyhedra matrix; this form is released when the insect cadaver disintegrates and is specifically adapted to infect the mid-gut epithelium of new larval hosts. The viral genome is a circular double-stranded DNA molecule that is packaged into a rigid, rod-shaped nucleocapsid about 250 nm in length. Because baculoviruses replicate exclusively in arthropods and present no known risk to vertebrates, they are classified as Biosafety Level 1 agents in most jurisdictions, making them both powerful tools for insect biocontrol and safe vectors for recombinant protein expression.
Baculovirus Primer-Our Molecular Delivery Truck
So, what exactly is our vector? Baculoviruses are exclusively pathogenic to arthropods, primarily insects, making them extremely safe for mammalian systems – a significant biosafety advantage. The workhorse species used in BEVS is Autographa californica multiple nucleopolyhedrovirus (AcMNPV). Its key features include a large genome (~134 kbp), which provides ample capacity for inserting large or multiple foreign genes, and its ability to efficiently infect established insect cell lines in suspension culture. Crucially, baculoviruses are non-replicative in mammalian cells, adding another layer of safety.
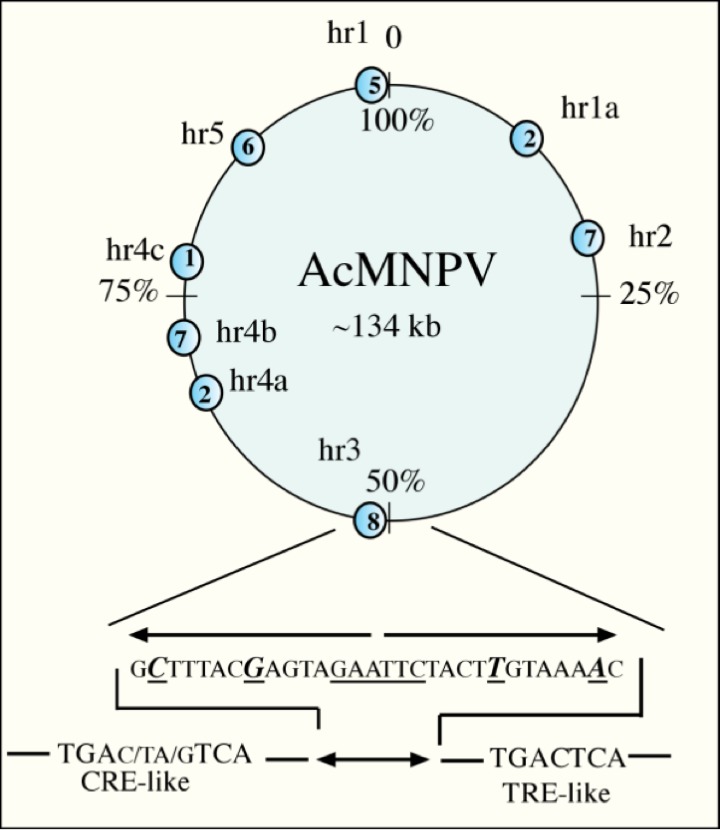 Fig3. Diagram of the AcMNPV
Fig3. Diagram of the AcMNPVThe Baculovirus Replication Cycle: Hijacking the Host
Understanding how the virus works is key to using it effectively. The infection cycle has two distinct phases, driven by different promoters:
Early Phase (0-6 hours post-infection): Immediately after entry, the virus uses its own RNA polymerase to express early genes necessary for DNA replication. This phase establishes the infection.
Late & Very Late Phase (6-72+ hours post-infection): As viral DNA replication kicks in, the virus transitions to using a virus-modified RNA polymerase. This drives the expression of late genes, primarily structural proteins for new virus particles (budded virus or BV). Finally, the very late phase is dominated by the incredibly strong promoters of the polyhedrin (polh) and p10 genes. These promoters are hyper-activated to produce massive quantities of occlusion body proteins in nature. In BEVS, we hijack these very late promoters (especially polh) to drive the expression of *our* recombinant protein of interest, often achieving yields far exceeding the cell's own protein production.
The polyhedrin promoter is virtually silent until the cell has already produced enough virus to survive, making it perfect for high-yield foreign protein expression without burdening early replication.
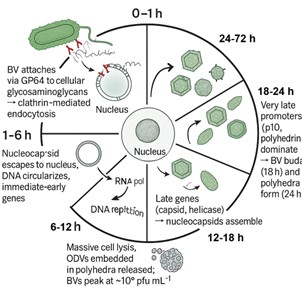 Fig4. The Baculovirus Replication Cycle
Fig4. The Baculovirus Replication CycleBaculovirus as a Vector: Engineering the Workhorse
How do we turn this virus into a protein factory? We use recombinant DNA technology:
The Transfer Plasmid: first,choose a transfer plasmid, pFastBac, pOET, pBAC, or modern Golden Gate versions. Then we create a small bacterial plasmid containing our gene of interest (GOI), flanked by baculovirus DNA sequences homologous to the region where we want to insert it (usually the polh or p10 locus). Crucially, the GOI is placed under the control of a very strong baculovirus promoter (like polh or p10 promoter). Add secretion signal (gp67, melittin, BiP) or keep cytosolic.
Co-transfection: This transfer plasmid is introduced into insect cells alongside wild-type baculovirus DNA.
Homologous Recombination: Inside the cell, a natural cellular process swaps the wild-type gene (e.g., polh) in the viral DNA with our GOI cassette from the transfer plasmid.
Isolation & Amplification: Only recombinant viruses (lacking polh/p10, but having our GOI) will produce plaques that look different using blue-white selection, for example. We painstakingly isolate these recombinant plaques, purify the virus, and amplify it to create a high-titer stock.
Modern systems like Bac-to-Bac (using site-specific transposition in E. coli) or flashBAC (using linearized viral DNA with a lethal deletion repaired only by recombination with the transfer plasmid) significantly streamline and speed up this process, eliminating the need for plaque purification.
Cell Lines: The Protein Production Factories
We need compatible host cells. Several continuous insect cell lines are used, each with characteristics:
* Sf9 & Sf21: Derived from the fall armyworm Spodoptera frugiperda pupal ovarian tissue. Sf9 is the most widely used due to ease of culture and good protein yields. Grows well adherently and in suspension. Sf21 is similar, sometimes preferred for initial plaque assays.
* High Five (BTI-Tn-5B1-4): Derived from the cabbage looper Trichoplusia ni egg cells. Often cited for achieving significantly higher yields (sometimes 2-10x more) for secreted proteins compared to Sf lines. However, it can be slightly more fragile in suspension culture and prone to clumping. Requires careful optimization.
* Newer Lines: Engineered lines like ExpresSF+ (derived from Sf9) or Tni-FNL are optimized for serum-free suspension growth, improved robustness, and potentially better glycosylation patterns.
Comparing the Cell Lines: Choosing the Right Factory
* Yield: High Five often wins for secreted proteins; Sf9/Sf21 are robust and reliable for intracellular and membrane proteins.
* Growth: Sf9/Sf21 generally grow faster and are easier to adapt to serum-free suspension. High Five can be more sensitive.
* Glycosylation: All insect cells produce high-mannose or paucimannose N-glycans, different from complex mammalian glycans. However, subtle differences exist between lines, and engineered lines with mammalianized glycosylation pathways are available (e.g., Mimic™ Sf9, SfSWT-1, Tni-FNL glycoengineered).
* Ease of Use: Sf9 is often the easiest starting point.
* Application: Choice depends on the protein (secreted/intracellular/membrane), yield needs, and required post-translational modifications (PTMs).
| Parameter | Sf9 | Sf21 | Tni-FNL | SfSWT-1 |
|---|---|---|---|---|
| Max density | 1.5×10⁷ | 1.2×10⁷ | 1.0×10⁷ | 1.3×10⁷ |
| Doubling time (h) | 20–24 | 18–22 | 18–20 | 22–24 |
| Secreted yield | Baseline | 20% | 3–5× | 2–3× |
| Glycosylation | paucimannose | paucimannose | paucimannose | complex, sialylated |
| Media cost | Low | Low | Medium | High |
| Regulatory | Gold std | Gold std | Gold std | Pre-IND |
Mechanism of BEVS: The Production Cascade
Let's put it all together:
1. Infection: We add the recombinant baculovirus stock (MVS) to a culture of healthy insect cells (e.g., Sf9 in suspension).
2. Virus Entry & Uncoating: The virus enters cells via receptor-mediated endocytosis, releases its DNA into the nucleus.
3. Gene Expression Cascade: Viral genes are expressed in phases (Early -> Late -> Very Late). Cellular machinery is progressively hijacked.
4. Recombinant Protein Production: During the very late phase, the powerful polh (or p10) promoter drives massive transcription massive transcription of your GOI.
5. Processing & Localization: Insect cells perform essential eukaryotic PTMs: folding (assisted by chaperones), disulfide bond formation, N-linked glycosylation (though simpler than mammalian), proteolytic cleavage, phosphorylation, acylation. Proteins can be directed intracellularly, secreted, or targeted to membranes based on signal sequences.
6. Harvest: Typically 48-72 hours post-infection, when recombinant protein production peaks and before significant cell lysis occurs (though lysis facilitates harvest for intracellular proteins). Cells/medium are harvested.
Comparison of Expression Systems: Why Choose BEVS?
BEVS sits uniquely between bacterial and mammalian systems:
* vs. Bacteria (E. coli): BEVS wins for complex, multi-domain, secreted, or glycosylated proteins that require eukaryotic folding and PTMs. E. coli often fails to fold complex proteins correctly, forms inclusion bodies, and lacks glycosylation. BEVS yields are typically lower than high-yielding E. coli processes but produce functional complex proteins.
* vs. Mammalian Cells (CHO, HEK): BEVS is generally faster (weeks vs. months for stable lines), cheaper (simpler media, no CO2), scales easily, and offers higher yields for many complex proteins. Mammalian cells provide human-like complex glycosylation crucial for therapeutic efficacy of some biologics. BEVS glycosylation is simpler but sufficient for many applications (research, diagnostics, vaccines, structural biology) and can be engineered.
| System | Speed (days) | Typical yield (mg L⁻¹) | PTMs | Cost (USD g⁻¹) | Scalability | Regulatory |
|---|---|---|---|---|---|---|
| E. coli | 3 | 100–1 000 | None | 10–50 | 10 000 L | GRAS |
| Yeast (Pichia) | 7 | 50–500 | Hyper-mannose | 100–300 | 100 000 L | GRAS |
| Plant transient | 7 | 10–100 | Plant glycans | 200–500 | Greenhouse | Emerging |
| CHO stable | 90–120 | 1–5 g L⁻¹ | Human-like | 200–1 000 | 20 000 L | Gold std |
| HEK293 transient | 10–14 | 50–500 | Human-like | 500–2 000 | 200 L | RUO |
| BEVS | 10–14 | 50–1 000 | Insect / human-like | 100–300 | 2 000 L | Gold std |
BEVS hits the sweet spot of speed, post-translational capability, and cost, especially for secreted, complex, or VLP-forming proteins.
Expression Systems or Platforms for Choice
-
E. coli -based system for rapid, high-yield protein production.
-
Combines bacterial and mammalian advantages for reliable protein expression.
-

Ideal for complex eukaryotic proteins using insect cell culture.
-
Closest to native state, perfect for high-value, functional proteins.
-

Fast and flexible, synthesizing proteins directly from DNA/RNA in vitro.
-

Serum-free medium for high-purity, animal-free protein production.
-

Transient expression for animal-free, high-quality recombinant proteins.
-

Cost-effective, high-yield production of recombinant proteins and peptides.
Advantages of the Baculovirus-Insect Cell System: The Compelling Case
* Eukaryotic Processing: Handles complex folding, disulfide bonds, essential PTMs (N-glycosylation, phosphorylation, acylation).
* High Yields: Particularly for complex proteins, often reaching 1-500 mg/L or more, especially with High Five for secreted proteins.
* Safety: Baculovirus is non-pathogenic to vertebrates and non-replicative in mammalian cells.
* Speed: Recombinant virus generation takes 1-3 weeks (faster with modern systems); protein production takes 3-7 days post-infection.
* Scalability: Insect cells grow well in suspension from small flasks to large bioreactors (1000L+).
* Capacity: Large genome accommodates very large genes or multiple genes (e.g., multi-subunit complexes, virus-like particles).
* Cost-Effectiveness: Lower medium costs and faster timelines than mammalian systems.
* Flexibility: Suitable for intracellular, secreted, and membrane proteins.
The Recombinant Protein Expression Process in BEVS: Step-by-Step
1. Gene Cloning: Clone your GOI into a baculovirus transfer vector (e.g., pFastBac1 for Bac-to-Bac).
2. Recombinant Bacmid Generation:
(Traditional): Co-transfect Sf9/Sf21 cells with transfer vector + viral DNA. Isolate recombinants via plaque assay (slow).
(Bac-to-Bac): Transform the transfer plasmid into E. coli containing the bacmid and helper plasmid. Transposition occurs, select white colonies. Isolate recombinant bacmid DNA.
(flashBAC): Co-transfect cells with transfer vector + flashBAC DNA (has a lethal deletion). Only recombinants survive.
3. Virus Stock Production: Transfect isolated bacmid DNA (Bac-to-Bac) or recombinant flashBAC DNA into Sf9 cells to produce P1 virus. Amplify P1 to generate high-titer P2 or P3 Master Virus Stock (MVS) in suspension culture.
4. Titer Determination: Quantify virus concentration (for example, Plaque Assay, TCID50, qPCR) to know infectious units per mL (IFU/mL).
5. Protein Production: Infect a culture of log-phase insect cells (Sf9, High Five) at the optimal Multiplicity of Infection (MOI) and cell density. Incubate for 48-72 hours.
6. Harvest: Centrifuge culture to separate cells (for intracellular/membrane proteins) from supernatant (for secreted proteins).
7. Analysis: Check expression via SDS-PAGE, Western Blot, activity assay.
8. Purification: Proceed with appropriate purification strategies (covered next).
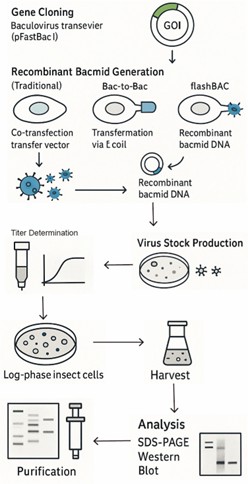 Fig5. The Recombinant Protein Expression Process in BEVS
Fig5. The Recombinant Protein Expression Process in BEVSApplications of BEVS: Where This Technology Shines
* Structural Biology: Producing milligram quantities of properly folded, post-translationally modified proteins for X-ray crystallography, NMR, Cryo-EM.
* Vaccine Development & Production: Virus-like particles (VLPs) for HPV, Hepatitis B, influenza, RSV, COVID-19 candidates. Subunit vaccines.
* Therapeutic Protein R&D: Enzymes, growth factors, cytokines, antibody fragments (scFv, Fab), soluble receptors. Often used for pre-clinical research and proof-of-concept.
* Diagnostic Reagents: Antigens for immunoassays, enzymes for diagnostic kits.
* Enzyme Production: Industrial enzymes requiring specific modifications.
* Gene Therapy Vectors: Engineering baculovirus itself as a gene delivery vehicle for mammalian cells.
* Multi-subunit Complexes: Expressing functional ion channels, receptors, chromatin complexes.
Proteins Suitable (and Less Suitable) for BEVS
Ideal Candidates:
* Large, complex multi-domain proteins.
* Proteins requiring disulfide bonds.
* Proteins requiring essential PTMs (N-glyc, phosphorylation, acylation).
* Secreted proteins and extracellular domains.
* Multi-subunit complexes and VLPs.
* Proteins toxic to bacterial or mammalian cells.
* Proteins where high yield of functional protein is critical.
Less Ideal Candidates:
* Proteins requiring mammalian-specific complex glycosylation for therapeutic function (unless using glycoengineered lines).
* Proteins requiring O-linked glycosylation (less common/robust in insect cells).
* Extremely high-volume, low-cost commodity proteins (bacteria or yeast might be better).
Optimization Strategies for BEVS: Maximizing Success
BEVS is powerful but not plug-and-play. Optimization is key:
Recombinant Expression Strategy:
* Promoter Choice: polh (strongest, most common), p10, hybrid promoters. Dual promoters for co-expression.
* Fusion Tags: His-tag (most common for IMAC purification), GST, FLAG, Fc-fusion (for secretion & purification, stability). Cleavage sites (TEV, Thrombin) for tag removal.
* Secretion Signals: Native signal sequence or heterologous signals (e.g., honeybee melittin, gp67) to direct protein to the supernatant.
Construct Design Strategy:
* Codon Optimization: Insect cells have codon bias; optimizing the GOI sequence can dramatically boost yield.
* Truncations/Domain Selection: Expressing only the functional domain can improve solubility/yield.
* Co-expression: Expressing chaperones (e.g., PDI, BiP) or complex partners simultaneously.
Purification Strategy:
* Clarification: Efficient removal of cells/cell debris (centrifugation, depth filtration).
* Capture: Affinity chromatography is king (IMAC for His-tag, Protein A/G for Fc-fusion, GST resin). For secreted proteins, often start with concentrated supernatant.
* Intermediate Purification: Ion exchange (IEX - AEX or CEX), Hydrophobic Interaction Chromatography (HIC).
* Polishing: Size Exclusion Chromatography (SEC - for monomer separation, buffer exchange), sometimes additional IEX.
* Concentration & Buffer Exchange: Ultrafiltration/Diafiltration (UF/DF).
Effect of Different Buffers on Purification:
* Lysis Buffers (Intracellular): Optimize pH, salt, detergent (for membrane proteins), protease inhibitors, reducing agents (for disulfide bonds). Avoid strong denaturants if native protein is needed.
* Binding/Wash Buffers (IMAC): pH, imidazole concentration, salt, additives (glycerol, mild detergents) to reduce non-specific binding & improve purity. Competitive elution with imidazole or pH shift.
* IEX Buffers: Choice of ion (e.g., NaCl vs. KCl), pH (determines charge), gradient slope. Critical for resolving isoforms.
* SEC Buffer: Choice affects protein stability, aggregation, and column resolution (e.g., PBS, Tris, HEPES; salt concentration; pH; potential additives like arginine to prevent aggregation).
Overall Strategy Optimization:
* Cell Line: Sf9 vs. High Five vs. glycoengineered? Test!
* Infection Parameters: MOI (typically 0.1-5), Cell Density at Infection (CDI - typically 1.5-3.0 x 10^6 cells/mL), Harvest Time (48-96 HPI - determine empirically).
* Culture Conditions: Serum-free vs. serum-containing media (serum-free preferred), temperature (27-28°C standard, sometimes lower temps for difficult proteins), agitation/aeration, pH control (in bioreactors).
* Analytics: Use SDS-PAGE, Western, activity assays, SEC-HPLC, MS throughout to guide optimization.
Recent Advances: Pushing the Boundaries
* Glycoengineered Cell Lines: SfSWT-1, Mimic™ Sf9, Tni-FNL glycoengineered: Engineered to produce proteins with human-like complex, biantennary N-glycans (reduced immunogenicity for therapeutics).
* Improved Vector Systems: More efficient recombination (flashBAC NEXTGEN), tighter regulation (inducible systems under development), vectors for CRISPR/Cas9 editing in insect cells.
* Cell Line Engineering: Lines engineered for enhanced productivity, apoptosis resistance, specific PTMs (e.g., tyrosine sulfation), or altered metabolism.
* High-Throughput & Automation: Automated platforms for cloning, virus generation, and small-scale expression screening (e.g., in 24-well or 96-deep well plates).
* Process Intensification: Perfusion cultures, high-cell density infections, improved bioreactor feeding strategies to boost titers and yields.
* BacMam Evolution: Enhanced baculovirus vectors for more efficient transduction and sustained expression in mammalian cells for research and gene therapy applications.
Challenges: Areas for Continued Improvement
* Glycosylation: While glycoengineering helps, achieving truly human-like, consistent glycosylation profiles at large scale remains a challenge and area of active research. Glycan heterogeneity can occur.
* Proteolysis: Insect cells express proteases, especially late in infection or during lysis. Strategies include using protease-deficient cell lines, adding broad-spectrum protease inhibitor cocktails, optimizing harvest time, lowering temperature, and designing less susceptible constructs.
* Scalability of Complex Processes: Scaling up production of membrane proteins or very large complexes while maintaining functionality and homogeneity can be difficult. Bioreactor optimization is key.
* Cost for Large-Scale Therapeutics: While cheaper than mammalian cells, BEVS can still be more expensive than microbial systems for very high-volume products. Process optimization drives cost down.
* Cell Lysis & Debris: Handling large volumes of lysate containing DNA and cellular debris requires efficient clarification strategies.
* Batch-to-Batch Variability: Ensuring consistency, especially for therapeutic applications, requires rigorous process control and analytics.
* Regulatory Path: While established for vaccines, the regulatory pathway for BEVS-produced complex glycoprotein therapeutics using glycoengineered lines is still maturing compared to CHO cells.
Conclusion
The Baculovirus-Insect Cell Expression System is a remarkably powerful and versatile platform. Its unique ability to produce high yields of complex, functionally active eukaryotic proteins with essential post-translational modifications, combined with its speed, scalability, and safety profile, ensures its continued relevance across research, diagnostics, and biomanufacturing – most notably in the life-saving vaccines many of us have received. While challenges like glycosylation control and proteolysis persist, ongoing advances in cell engineering, vector design, and process optimization are continually expanding its capabilities. Whether you're producing antigens for research, VLPs for vaccines, or exploring novel therapeutics, BEVS offers a robust and proven solution.
Related Services
- Insect Expression
- Bacterial Expression
- Yeast Expression
- Mammalian Expression
- Cell-Free Expression
- Serum-Free System
- Nicotiana Tabacum Platform
- Rice Endosperm Platform
Related Resources
-
How Insect Cells Turbocharge Protein Production
-
Unlocking BEVS: The Secret to Efficient Protein Production!
-
Unlock the Perfect Protein Expression System for Your Research
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

