Recombinant HBeAg protein, His-tagged
| Cat.No. : | CDE017 |
| Product Overview : | Recombinant hepatitis B virus “e” antigen(10-149a.a) fused with an C-terminal His tag was expressed in E. coli. The HBeAg sequence is derived from HBV adw2. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | HBV |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | 10-149 a.a. |
| Description : | Hepatitis B virus is the main cause for human liver disease, chronic infection frequently causes liver cancer and cirrhosis. The HBV core gene codes 2 distinct protein products, a 21.5-kDa protein being assembled to form nucleocapsid particles designated HBcAg, which wraps the viral DNA as well as the viral polymerase and RNase H, and a precore protein, designated as HBeAg, which is directly to the endoplasmic reticulum, processed at N- and C-terminally and secreted as non-particulate e-anitgen (HBeAg). The pre-core protein contains an extra 29 N-terminal amino acids, serving as a signal peptide to direct the nascent polypeptide into secretory pathway. After the secretion, mature HBeAg is deleted at the residue 149 C-terminally and retains 10 precore residues N-terminally. HBcAg and HBeAg are distinctly recognized by antibodies but highly cross-reactive at the T-cell level. The e antigen is found in the circulation, it is found during the active HBV infection, positive result indicates the risk for contagiousness, and is also used as indicator for the effectiveness of HBV treatment. Positive anti-HBeAg specifies an active stage of acute HBV infection that is in its final stages, the risk for contagiousness is dramatically reduced. |
| Form : | Phosphate buffered saline with 50mM arginine. |
| Molecular Mass : | 23kDa |
| AA Sequence : | SKLCLGWLWG MDIDPYKEFG ATVELLSFLP SDFFPSVRDL LDTASALYRE ALESPEHCSP HHTALRQAIL CWGELMTLAT WVGNNLEDPA SRDLVVNYVN TNVGLKIRQL LWFHISCLTF GRETVLEYLV SFGVWIRTPP AYRPPNAPIL STLPETTVVR RRDRGRSPRR RTPSPRRRRS PSPRRRRSQS RESQC. |
| Purity : | Protein is >95% pure as determined by 12% PAGE (coomassie staining). |
| Applications : | Immunoassay |
| Storage : | HBeAg although stable at 4 centigrade for 1 week, should be stored below -18 centigrade. Please prevent freeze thaw cycles. |
| ◆ Recombinant Proteins | ||
| HBcAg-19H | Recombinant Hepatitis B Virus C protein | +Inquiry |
| HCV-02 | Recombinant HCV Core protein | +Inquiry |
| C-2929H | Recombinant Hepatitis B virus genotype A2 subtype adw2 C protein, His-SUMO-tagged | +Inquiry |
| CD200-06H | Active Recombinant Human CD200 Protein, His-tagged | +Inquiry |
| C-0839H | Recombinant HBV C Protein (Met1-Val149), C-His tagged | +Inquiry |
IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
Journal: PLoS Pathogens PubMed ID: 25503988 Data: 2014/12/1
Authors: Adrian Egli, Deanna M. Santer, Michael Gale
Article Snippet:Supernatants from stimulated PBMC cultures (day 7; diluted 1∶2) were added and the amount of bound antibody was determined as above except supernatants were incubated overnight to increase sensitivity.Supernatants from stimulated PBMC cultures (day 7; diluted 1∶2) were added and the amount of bound antibody was determined as above except supernatants were incubated overnight to increase sensitivity.. To confirm specificity, supernatants were added to plates coated with hepatitis B virus surface antigen (Creative BioMart) or HCV E2 antigen (Immunodiagnostics, Inc.) and no signal was detected above background (Median ODs for HA coated wells: 0.607; HBs Ag: 0.00625; HCV E2: 0.00425; and unstimulated sample supernatant with HA coated wells: 0.01175).. Results are expressed as absorbance values (405 nm–570 nm) with the plate blank subtracted.Results are expressed as absorbance values (405 nm–570 nm) with the plate blank subtracted.
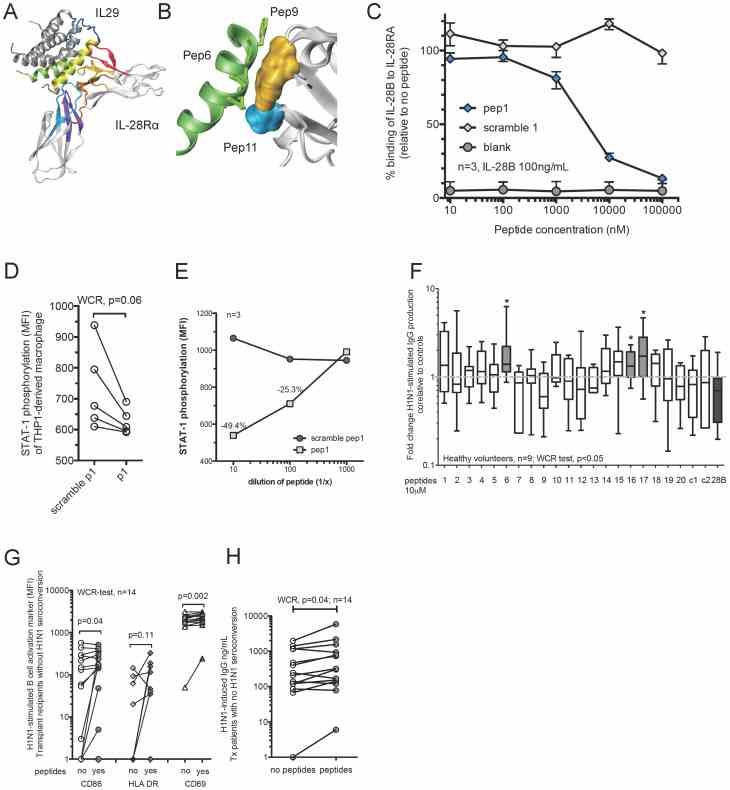
(A) Design of inhibitory peptides based on the crystal structures of IL-29 and the IL28RA. A computer prediction of the interaction between IL-29 and the IL28RA is illustrated. Colored fragments represent proposed sites of significant interaction using a proximity model. (B) Example for detailed in silico interaction between peptide 6 and IL-28Rα (peptides 9 and 11). (C) Inhibitory activity of antagonistic peptide 1 against IL-28B binding to IL28RA. ELISA was used to measure the binding of a fixed concentration of IL-28B (100 ng/mL) to IL28RA challenged by increasing concentrations of the inhibitory peptide and control peptide (scrambled version). The data shown is representative of three independently repeated experiments. Whiskers indicate the interquartile range. (D) STAT1 phosphorylation in THP1-derived macrophages treated with peptide 1 and challenged with recombinant IL-28B (100 ng/mL) for 15 min in comparison to scramble peptide control. Data of five individual experiments is shown. Wilcoxon matched-pairs signed rank (WCR)-test was used. (E) STAT1 phosphorylation in THP1-derived macrophages treated with different doses of peptide 1 and challenged with recombinant IL-28B (100 ng/mL) for 15 min in comparison to scramble peptide 1 control. Symbols represent median of three independently repeated experiments. (F) PBMCs from healthy volunteers were pre-treated with peptides for two hours prior to 5-day stimulation with H1N1. In addition recombinant IL-28B (28B) and control peptides (c1, SV40-based peptide; c2, a duck
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All C Products
Required fields are marked with *
My Review for All C Products
Required fields are marked with *



