Recombinant Human SIRT2
| Cat.No. : | SIRT2-30564TH |
| Product Overview : | Full-length recombinant Human SIRT2 with an N-terminal tag. MWt 64kDa. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | Non |
| Protein Length : | 389 amino acids |
| Description : | This gene encodes a member of the sirtuin family of proteins, homologs to the yeast Sir2 protein. Members of the sirtuin family are characterized by a sirtuin core domain and grouped into four classes. The functions of human sirtuins have not yet been determined; however, yeast sirtuin proteins are known to regulate epigenetic gene silencing and suppress recombination of rDNA. Studies suggest that the human sirtuins may function as intracellular regulatory proteins with mono-ADP-ribosyltransferase activity. The protein encoded by this gene is included in class I of the sirtuin family. Several transcript variants are resulted from alternative splicing of this gene. |
| Molecular Weight : | 64.000kDa inclusive of tags |
| Tissue specificity : | Widely expressed. Highly expressed in heart, brain and skeletal muscle, while it is weakly expressed in placenta and lung. Down-regulated in many gliomas suggesting that it may act as a tumor suppressor gene in human gliomas possibly through the regulatio |
| Form : | Liquid |
| Storage buffer : | Preservative: NoneConstituents: 25% Glycerol, 50mM Tris HCl, 150mM Sodium chloride, 10mM Glutathione, 0.25mM DTT, 0.1mM EDTA, 0.1mM PMSF, pH 7.5 |
| Storage : | Shipped on dry ice. Upon delivery aliquot and store at -80oC. Avoid freeze / thaw cycles. |
| Sequence Similarities : | Belongs to the sirtuin family.Contains 1 deacetylase sirtuin-type domain. |
| Gene Name | SIRT2 sirtuin 2 [ Homo sapiens ] |
| Official Symbol | SIRT2 |
| Synonyms | SIRT2; sirtuin 2; SIR2L, sirtuin (silent mating type information regulation 2 homolog) 2 (S. cerevisiae) , sirtuin (silent mating type information regulation 2, S.cerevisiae, homolog) 2; NAD-dependent deacetylase sirtuin-2; |
| Gene ID | 22933 |
| mRNA Refseq | NM_001193286 |
| Protein Refseq | NP_001180215 |
| MIM | 604480 |
| Uniprot ID | Q8IXJ6 |
| Chromosome Location | 19q13 |
| Pathway | Signaling events mediated by HDAC Class I, organism-specific biosystem; Signaling events mediated by HDAC Class III, organism-specific biosystem; |
| Function | NOT NAD+ ADP-ribosyltransferase activity; NAD+ binding; NAD-dependent histone deacetylase activity; histone acetyltransferase binding; histone deacetylase binding; |
| ◆ Recombinant Proteins | ||
| SIRT2-463H | Recombinant Human SIRT2, His-tagged | +Inquiry |
| SIRT2-5759H | Recombinant Full Length Human SIRT2 Protein (Met1-Gln389), N-GST tagged | +Inquiry |
| SIRT2-174H | Recombinant Human SIRT2 protein, T7/His-tagged | +Inquiry |
| SIRT2-30566TH | Recombinant Human SIRT2, His-tagged | +Inquiry |
| SIRT2-191H | Recombinant Human SIRT2 Protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| SIRT2-1834HCL | Recombinant Human SIRT2 293 Cell Lysate | +Inquiry |
NQO1 regulates mitotic progression and response to mitotic stress through modulating SIRT2 activity
Journal: Free radical biology & medicine PubMed ID: 30114477 Data: 2019/10/1
Authors: Hong-Jun Kang, Ha Yong Song, Athanassios Vassilopoulos
Article Snippet:Recombinant human SIRT2 and GST tagged NQO1 (GST-NQO1) proteins were purchased from Abcam and Creative BioMart, respectively.. SIRT2 and GST-NQO1 were incubated for 1 hour at 4°C with rotation in 20 mM Tris-HCl (pH 7.62), 150 mM NaCl and 1% Triton X-100 binding buffer.SIRT2 and GST-NQO1 were incubated for 1 hour at 4°C with rotation in 20 mM Tris-HCl (pH 7.62), 150 mM NaCl and 1% Triton X-100 binding buffer.
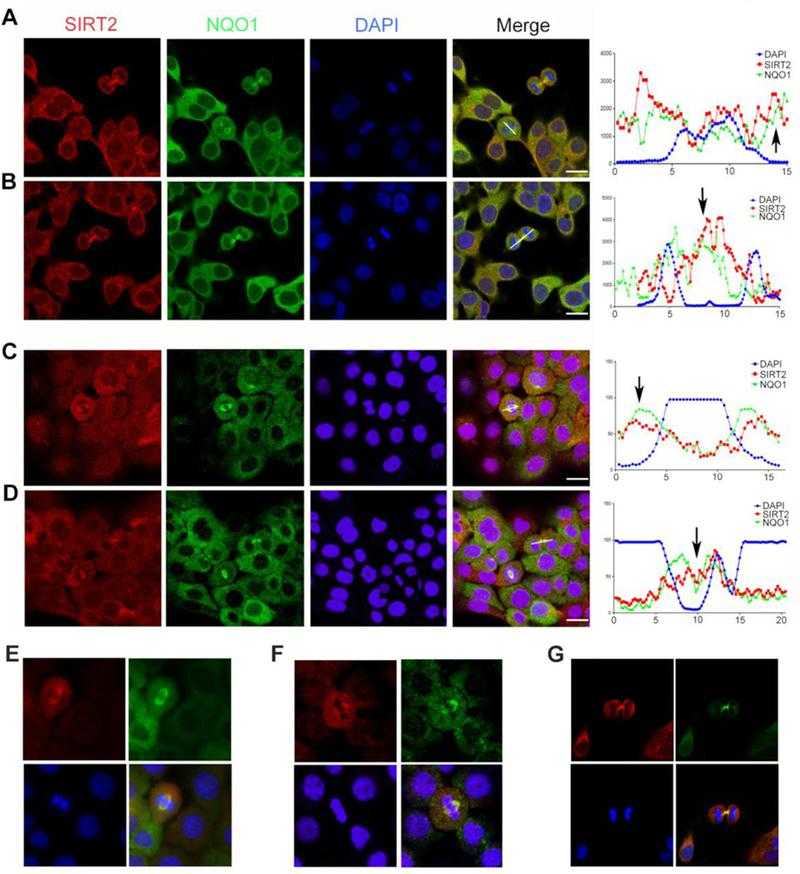
NQO1 and
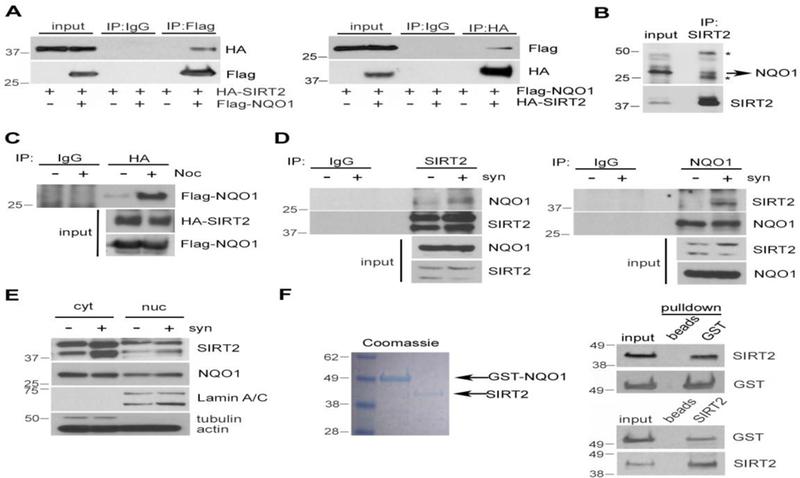
NQO1 interacts directly with
![NQO1 positively regulates SIRT2 activity. (A) MCF-7 cells were transfected with either control (ctr) siRNA or NQO1 siRNA. 48 h after transfection, lysates were analyzed by western blotting using antibodies against Lys-40 acetylated tubulin (Ac-Tubulin), NQO1, SIRT2 and tubulin/actin. Quantification of acetylated tubulin levels are presented, **p<0.01. (B) MDA-MB-231 cells (NQO1 null) were transfected with either empty vector (ctr), Flag-NQO1 or Flag-NQO1P187S. 48h after transfection, lysates were analyzed by western blotting using antibodies against Lys-40 acetylated tubulin (Ac-Tubulin), Flag, SIRT2 and tubulin/actin. Quantification of acetylated tubulin levels are presented, **p<0.01. (C) MCF-7 cells were treated with Dicoumarol (100 uM) for different times or with different concentrations as indicated for 24h. Lysates were analyzed by western blotting using antibodies against Lys-40 acetylated tubulin (Ac-Tubulin), SIRT2 and tubulin. Quantification of acetylated tubulin levels are presented, *p<0.05. (D, E, F) MCF-7 cells were transfected with either control (ctr) siRNA or NQO1 siRNA. Cells were lysed in extraction buffer, and NADtotal (NAD and NADH) (D) as well as NADH alone (E) were calculated. The ratio of NAD/NADH (F) is calculated based on the formula (NADtotal-NADH)/NADH. Data represent Mean ± SEM of three independent experiments, *P<0.05. (G) MCF-7 cells were transfected with either control (ctr) siRNA or NQO1 siRNA. 48 h after transfection, SIRT2 activity in these lysates was determined by using a fluorometric assay based on the deacetylation of a unique target peptide included in the kit (FLUOR DE LYS? SIRT2 Deacetylase Fluorometric Assay Kit). Data represent Mean ± SEM of three independent experiments, *P<0.05. (H) MCF-7 cells were transfected with either control (ctr) siRNA or NQO1 siRNA. NQO1 knockdown cells were next transfected with NQO1 cDNA or treated with NAD+ (0.5 mM). Cell extracts were analyzed by western blotting using antibodies against Acetylated-Tubulin (Ac-tubulin), NQO1, SIRT2 and actin/GAPDH. * denotes exogenously expressed Flag-NQO1, whereas ** denotes the expression of endogenous NQO1. (I) MCF-7 cells were transfected with either control (ctr) siRNA or SIRT2 siRNA in the presence or absence of exogenously expressed NQO1 or NQO1P187S. Cell extracts were analyzed by western blotting using antibodies against acetylated histone H4 lysine16 (Ac-H4K16), SIRT2, Flag (NQO1) and actin. Note that NQO1P187S cannot be detected as this mutation affects enzymatic activity by extremely decreasing stability due to ubiquitination and proteasomal degradation [44]. (J) Wild-type (Sirt2+/+) and Sirt2 knockout (Sirt2-/-) MEFs were transfected with either control (ctr) siRNA, Nqo1 siRNA or Flag-NQO1 cDNA. Cell extracts were analyzed by western blotting using antibodies against acetylated histone H4 lysine16 (Ac-H4K16), SIRT2, Flag (NQO1) and actin.](productimages/extendimages/pmc06170003__nihms-1506327-f0004.jpg)
NQO1 positively regulates
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All SIRT2 Products
Required fields are marked with *
My Review for All SIRT2 Products
Required fields are marked with *



