B Cell Receptor-Associated Proteins
Related Symbol Search List
- BANK1
- BLK
- BLNK
- BTK
- CD19
- CD22
- CD5
- CD79A
- CD79B
- CD81
- CR2
- FCRL1
- FCRL2
- FCRL3
- FCRL4
- FCRL5
- FCRL6
- FYN
- LAX1
- Lyn
- NFAM1
- CD45
Immunology Background
Background
About B Cell Receptor-Associated Proteins
B cell receptor-associated proteins are a group of molecules that are closely associated with the B cell receptor (BCR) complex. The BCR is a membrane-bound immunoglobulin (antibody) molecule expressed on the surface of B cells. It plays a crucial role in the recognition and binding of specific antigens, initiating the activation and immune response of B cells.
The BCR is composed of two main components: the membrane-bound immunoglobulin molecules and the associated signaling proteins. The signaling proteins that are associated with the BCR include Igα (CD79a) and Igβ (CD79b), which form a heterodimer known as the B cell antigen receptor complex (BCR complex). CD79a/CD79b heterodimeric unit contains three domains (extracellular, transmembrane, and intracellular) and is bound together with disulfide bonds formed between cysteine residues of its extracellular domains (Fig.1). These proteins are non-covalently associated with the immunoglobulin heavy and light chains of the BCR.
The BCR-associated proteins are critical for several key functions in B cell activation and immune responses. They serve as scaffolds for the recruitment and activation of signaling molecules, including kinases and adaptor proteins, which transmit the signals from the BCR to the nucleus. These signals regulate various cellular processes, such as B cell proliferation, differentiation, survival, and antibody production.
Notably, mutations or deficiencies in BCR-associated proteins, such as Igα and Igβ, can lead to impaired B cell function and immune deficiencies. For example, mutations in the Igα or Igβ genes can cause a rare primary immunodeficiency disorder called agammaglobulinemia, characterized by a lack of mature B cells and reduced antibody production.
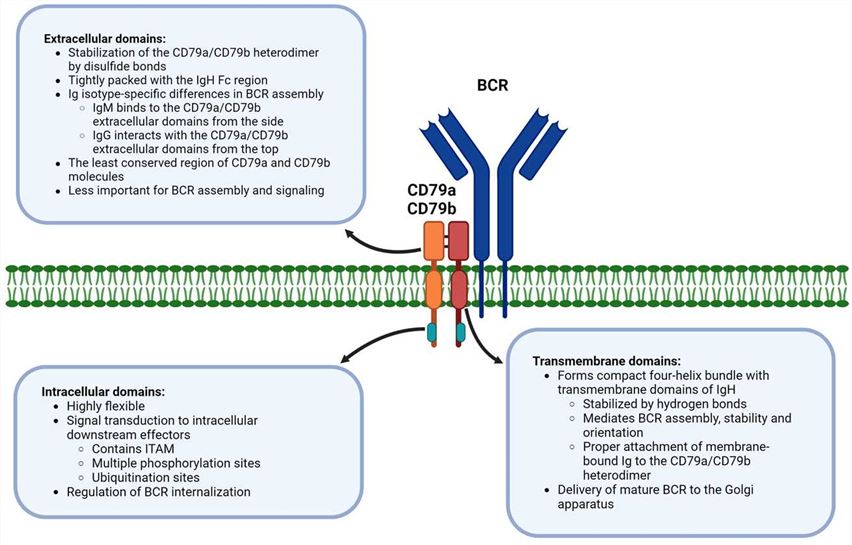 Fig.1 Distinct function of CD79a/CD79b domains in B-cell receptor signaling. (Tkachenko A, et al., 2024)
Fig.1 Distinct function of CD79a/CD79b domains in B-cell receptor signaling. (Tkachenko A, et al., 2024)Importance of BCR-Associated Proteins
BCR-associated proteins play a crucial role in B cell development, differentiation, and activation. Here are some key aspects highlighting their importance:
| Key roles | Importance |
|---|---|
| B Cell Development |
|
| B Cell Activation and Signaling |
|
| Antibody Production |
|
| Immune Responses and Defense |
|
| Autoimmunity and Tolerance |
|
In summary, BCR-associated proteins are indispensable for B cell development, differentiation, and activation. They enable B cells to recognize and respond to specific antigens, initiate signaling cascades, promote antibody production, and contribute to immune responses and defense mechanisms. Dysfunction or alterations in these proteins can lead to immunodeficiency, impaired immune responses, or dysregulated immune reactions, highlighting their vital role in maintaining a healthy immune system.
B Cell Receptor-Associated Proteins
Key Molecules and Functions Associated with BCR Activation
| Key molecules | Functions |
|---|---|
| BANK1 (B-cell scaffold protein with ankyrin repeats 1) |
|
| BLK (B lymphocyte kinase) |
|
| BLNK (B cell linker protein) |
|
| BTK (Bruton's tyrosine kinase) |
|
| CD19 |
|
| CD22 |
|
| CD5 |
|
| CD79A and CD79B |
|
| CD81 |
|
| CR2 (Complement receptor 2) |
|
| FCRL1, FCRL2, FCRL3, FCRL4, FCRL5, FCRL6 (Fc receptor-like proteins) |
|
| FYN (Fyn tyrosine kinase) |
|
| LAX1 (Lymphocyte transmembrane adaptor 1) |
|
| Lyn (Lyn tyrosine kinase) |
|
| NFAM1 (Adaptor protein NFAT-activating molecule 1) |
|
| CD45 |
|
These proteins, and others, collectively contribute to the complex signaling pathways and interactions involved in B cell receptor signaling, activation, and immune responses.
Key Signaling Pathways and Molecular Interactions Involved in BCR Activation
The activation of the B cell receptor (BCR) triggers a series of signaling pathways and molecular interactions that lead to B cell activation, proliferation, and antibody production. Here are some key signaling pathways and molecular interactions involved in BCR activation:
| Key signaling pathways | Molecular interactions |
|---|---|
| BCR Crosslinking |
|
| Immunoreceptor Tyrosine-Based Activation Motifs (ITAMs) |
|
| Lyn Kinase Activation |
|
| Syk Kinase Recruitment and Activation |
|
| Phospholipase Cγ2 (PLCγ2) Activation |
|
| Calcium Signaling |
|
| Protein Kinase Activation |
|
| BCR-Associated Scaffold Proteins |
|
| Ras-MAPK Pathway |
|
| PI3K-Akt Pathway |
|
| Nuclear Factor-κB (NF-κB) Pathway |
|
| Transcriptional Regulation |
|
These signaling pathways and molecular interactions collectively contribute to BCR activation and subsequent B cell responses, including antibody production, class switching, affinity maturation, and memory B cell formation.
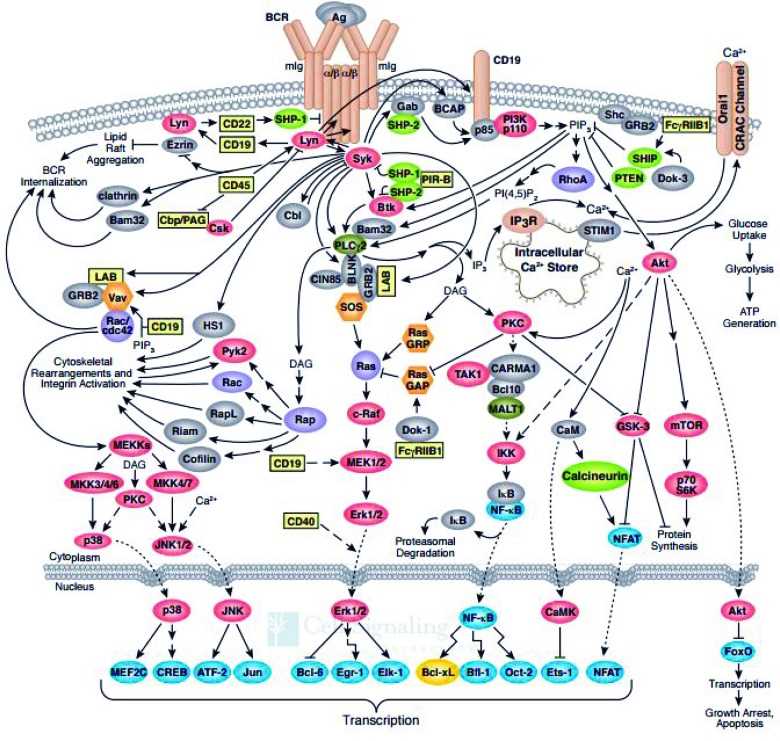 Fig.2 B-cell receptor signaling pathway. (Merolle MI, et al., 2018)
Fig.2 B-cell receptor signaling pathway. (Merolle MI, et al., 2018)Diseases and Disorders Associated with Dysregulation of BCR-Associated Proteins
Dysregulation of BCR-associated proteins can contribute to various diseases and disorders. Here are some examples:
Immunodeficiencies
- Mutations in BCR-associated proteins, such as CD79a, CD79b, or Ig chains, can lead to primary immunodeficiencies.
- These immunodeficiencies result in impaired B cell development, reduced BCR signaling, and compromised antibody production, leading to increased susceptibility to infections.
- Examples include agammaglobulinemia, common variable immunodeficiency (CVID), and selective IgA deficiency.
Autoimmune Diseases
- Dysregulation of BCR-associated proteins can contribute to the development of autoimmune diseases, where the immune system mistakenly attacks self-tissues.
- Aberrant signaling through BCR-associated proteins can lead to the production of autoantibodies targeting self-antigens.
- Examples of autoimmune diseases associated with B cell dysregulation include systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome.
B Cell Malignancies
- Alterations in BCR-associated proteins can contribute to the development of B cell malignancies, including B cell lymphomas and leukemias.
- Mutations or chromosomal translocations involving BCR-associated proteins, such as IgH or CD79a/b, can result in constitutive BCR signaling and uncontrolled B cell proliferation.
- Examples include Burkitt lymphoma, diffuse large B cell lymphoma (DLBCL), and chronic lymphocytic leukemia (CLL).
Hyperactive B Cell Signaling
- Dysregulation of BCR-associated proteins can lead to hyperactive BCR signaling, resulting in excessive B cell activation and antibody production.
- This dysregulation can contribute to hypergammaglobulinemia, polyclonal B cell activation, and antibody-mediated disorders.
- Examples include hyper-IgM syndrome, certain forms of monoclonal gammopathy, and Waldenström macroglobulinemia.
Lymphoproliferative Disorders
- Dysregulated BCR signaling can contribute to the development of lymphoproliferative disorders characterized by abnormal B cell expansion.
- Aberrant BCR signaling can promote uncontrolled B cell proliferation and survival.
- Examples include lymphoproliferative disorders associated with chronic active Epstein-Barr virus (EBV) infection and hairy cell leukemia.
It's important to note that the dysregulation of BCR-associated proteins can occur through various mechanisms, including genetic mutations, chromosomal abnormalities, altered expression levels, and post-translational modifications. The resulting impact on B cell function and immune responses can vary depending on the specific protein involved and the nature of the dysregulation.
Case Study
Case 1: Jahn L, Hombrink P, Hassan C, et al. Therapeutic targeting of the BCR-associated protein CD79b in a TCR-based approach is hampered by aberrant expression of CD79b. Blood. 2015;125(6):949-958.
CD79b-specific T-cell clones K308 and S100 were assessed for their potency to recognize a variety of primary B-cell malignancies and malignant B-cell lines.
Clones K308 and S100 efficiently recognized HLA-A2–positive ALL cell lines (A). Clone S100 also recognized 4 HLA-A2–positive primary CLL samples (B). In addition, stimulation with primary HLA-A2–positive CLL and ALL samples resulted in strong IFN-γ production of clone K308 (C-D). No recognition of the HLA-A2–negative CLL sample ERV 8300 was observed (D). Coincubation of the ALL cell lines or primary HLA-A2–positive ALL and CLL samples with clone K308 resulted in efficient lysis of the malignant cells (E-F), indicating a cytotoxic effect of clone K308.
These results indicate efficient recognition of CD79b-expressing HLA-A2–positive B-cell malignancies by the CD79b-specific clones K308 and S100.
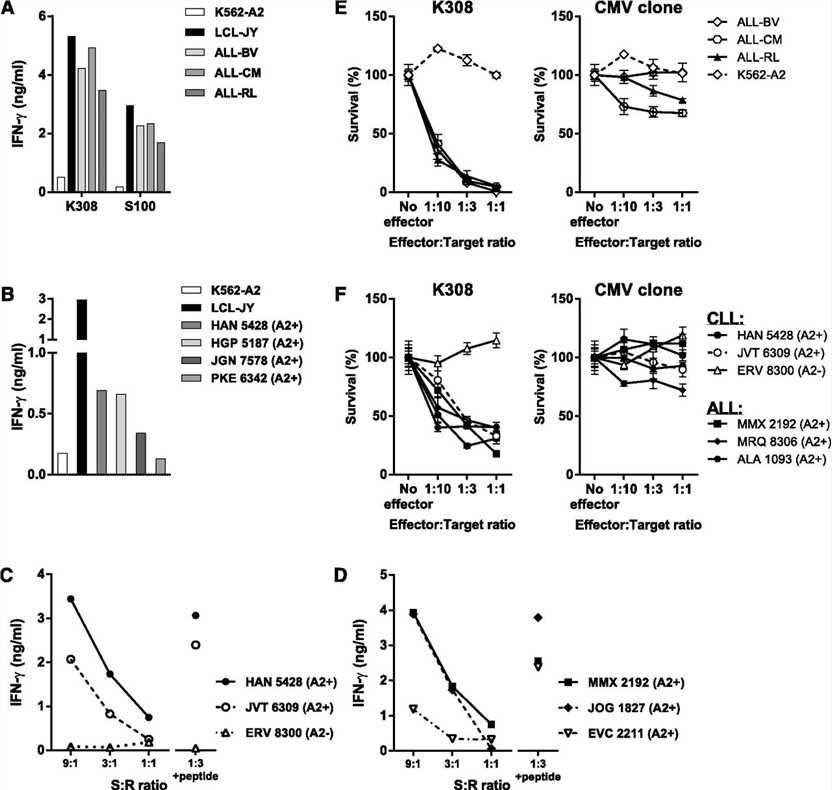 Fig.1 CD79b expression and shRNA knockdown confirm CD79b specificity of isolated T-cell clones.
Fig.1 CD79b expression and shRNA knockdown confirm CD79b specificity of isolated T-cell clones.Case 2: Huse K, Bai B, Hilden VI, et al. Mechanism of CD79A and CD79B support for IgM+ B cell fitness through B cell receptor surface expression. J Immunol. 2022;209(10):2042-2053.
To assess equivalent production of the rescue proteins, we linked CD79B to GFP using a 2A ribosome skipping peptide. Reintroduction of WT CD79B resulted in strong surface expression of CD79A, CD79B, and IgM, contrasting CD79B G137S, which led to low surface expression of the three BCR components (A). Importantly, the low surface expression levels observed with CD79B G137S were not due to lower retroviral transduction efficiency because both CD79B constructs showed similar GFP expression levels (A). In addition, CD79B mRNA expression levels were similar (B), whereas the total level of CD79B protein was higher for CD79B WT than CD79B G137S (C, D), suggesting that the mutant has reduced protein stability. However, the most striking difference was in protein maturation of CD79B (E).
These data demonstrate that CD79B protein governs the maturation and surface expression of both CD79A and IgM. The model that emerges from these results is that heterodimerization of CD79A and CD79B is required for proper protein maturation.
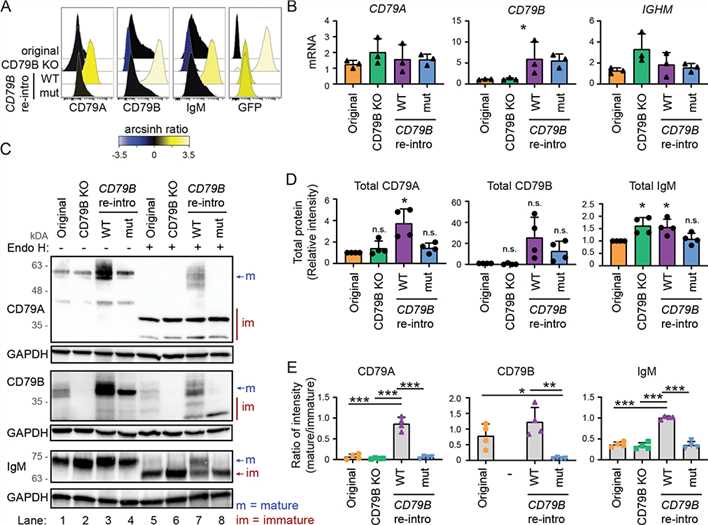 Fig.2 BCR surface expression and protein maturation in CD79B KO cells can be rescued by reintroducing CD79B.
Fig.2 BCR surface expression and protein maturation in CD79B KO cells can be rescued by reintroducing CD79B.References
- Merolle MI, Ahmed M, Nomie K, Wang ML. The B cell receptor signaling pathway in mantle cell lymphoma. Oncotarget. 2018;9(38):25332-25341.
- Tkachenko A, Kupcova K, Havranek O. B-cell receptor signaling and beyond: The Role of Igα (CD79a)/Igβ (CD79b) in normal and malignant B cells. International Journal of Molecular Sciences. 2024; 25(1):10.

