Rho GTPases
Related Symbol Search List
Immunology Background
What is Ras GTPase Family Proteins
Ras is the abbreviation of rat sarcoma. Ras protein is the expression product of the proto-oncogene c-ras. GTPases, together with their associated regulators and effectors, are central control elements which participate in signal transduction pathways that touch on almost every aspect of cell biology. Most of these proteins belong to the superfamily named for the RAS oncoprotein. RAS proteins exist in equilibrium between GTP- and GDP-bound forms. GEFs and GAPs regulate the relative amounts of each form. (Fig.1)
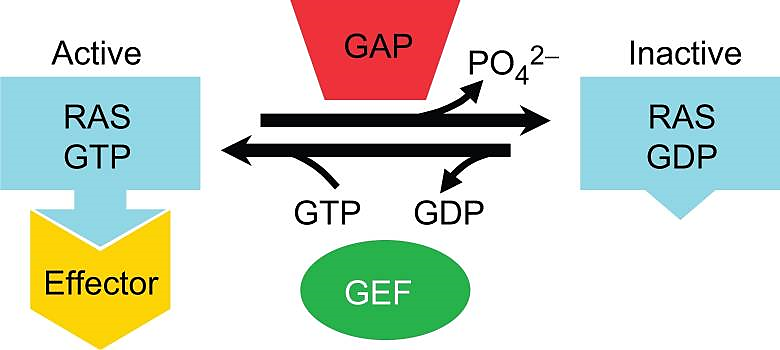 Figure 1. Equilibrium between GTP- bound RAS proteins and GDP-bound RAS proteins regulated by GEFs and GAPs
Figure 1. Equilibrium between GTP- bound RAS proteins and GDP-bound RAS proteins regulated by GEFs and GAPs
Function of Ras GTPase Family Proteins
The Ras GTPases serve as molecular switches, the timing of it modulated by a lot of factors. These factors include guanine nucleotide exchange factors (GEFs), which catalyze the transformation of Ras to the GTP-bound “on” state, and GTPase activating proteins (GAPs) promote the intrinsic rate of hydrolysis of bound GTP to GDP. The mechanism of the switching process involves a guanine-nucleotide-dependent conformational change in two discrete regions of the Ras protein, called the Switch I and Switch II domains. This structural rearrangement allows GTP-bound Ras to interact with and activate specific downstream targets or effector proteins. Such mutations in Ras cause the constitutive accumulation of the GTP-bound form are oncogenic.
The Ras superfamily of small GTPases contains a group of molecular switches that regulate a lot of cellular functions. The inclusion of the small GTPases is crucial for further understanding of mitogenesis, cytoskeletal organization, vesicle traffic, and nuclear transport components of the molecular machines that drive these processes. Varies downstream effectors, and interconnected networks of GTPase-regulated protein kinase cascades proved that the control mechanisms are very complex.
Regulation of Ras GTPase Family Proteins
According to homologous sequences and functional similarities, the small GTPase superfamily is divided into five major subfamilies, they are Ras, Rho, Rab, Ran and Arf (ADP ribosylation factor). The p120-RasGAP is activating protein that stimulated GTP hydrolysis of Ras-GTP to Ras-GDP in its role as a negative regulator of Ras. However, in cells expressing oncogenic Ras, p120-RasGAP helps as a transducer in other signal transduction pathways and plays a role in proliferation, migration, and anti- and pro-apoptosis that is independent of its GAP activity. The loss of RasGAP function plays a role in angiogenesis.Small GTPases show high-affinity for GDP and GTP, and with limited intrinsic GTP hydrolysis and GDP/GTP exchange activities. Two main types of protein regulate GDP/GTP cycling. Guanine-nucleotide-exchange factors (GEFs) accelerate formation of the active, GTP-bound form, and GTPase-activating proteins (GAPs) promote the intrinsic GTPase activity to form the inactive GDP-bound form. The two nucleotide-bound states have similar conformations but these have obvious differences between the corresponding switch I and switch II regions: the GTP-bound conformation has high affinity for effector targets. It is mainly via the changes of conformation in these two switches where regulatory proteins and effectors ‘sense’ the nucleotide status of the small GTPases. Arf proteins include additional N-terminal sequences, and Ran has additional C-terminal sequences that undergo significant conformational changes during GDP/GTP cycling. Although the GTP bound form is the active form for all Ras superfamily GTPases, the cycling between the GDP bound and GTP bound states, where the distinct functions are associated with each nucleotide-bound form, is also crucial for the activities of Rab, Arf and Ran GTPases. The core effector domain contains the switch I domain and is essential for direct association with the effectors.
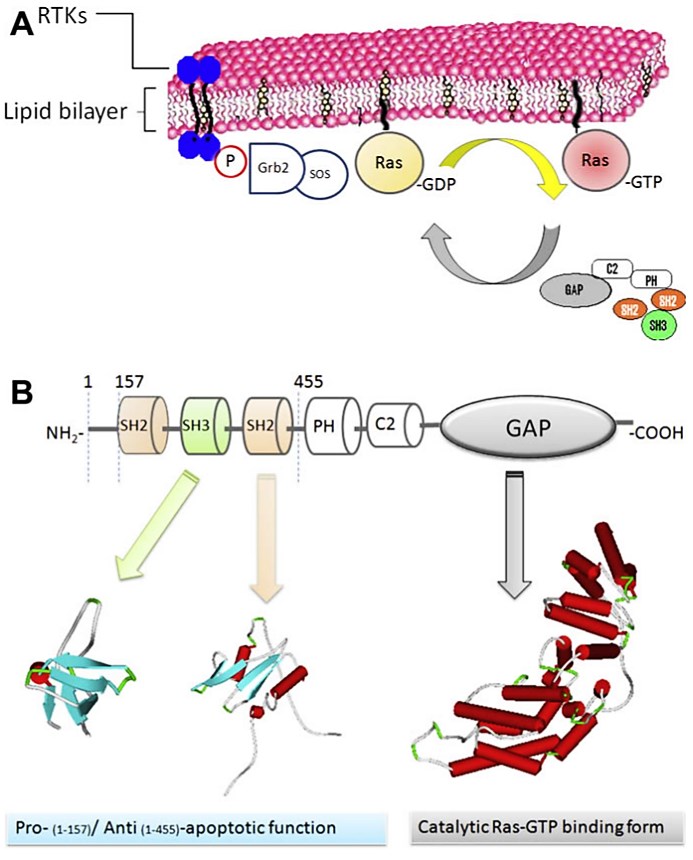 Figure 2. Ras cycle and RasGAP activities. (A) Ras cycle. (B) p120-RasGAP activities.
Figure 2. Ras cycle and RasGAP activities. (A) Ras cycle. (B) p120-RasGAP activities.
p120-Ras GTPase activating protein (RasGAP) is an important GTPase activating protein. P120-RasGAP is a down-regulator of downstream signaling through several receptors (plateletderived growth factor (PDGF), epidermal growth factor (EGF), ephrin, insulin, and colony-stimulating factor-1 (CSF1) in the cells of mammal. P120-RasGAP is an effector of Ras protein function, when its N-terminal region (N-GAP) triggers downstream signals of its GAP activity. This p120-RasGAP N-terminal region serves as a positive effector of Ras in tumor cells. The CaLB/C2, PH, SH2 and SH3 domains appear in this N-terminal part and involved in transmission or downstream signaling. p120-RasGAP has many binding partners. p120-RasGAP (Ras GTPase activating protein) is composed of several domains such as GAP (GTPase-activating proteins), PH (pleckstrin homology), CaLB/C2 (calcium-dependent phospholipid-binding domain), Src Homology 3 (SH3), Src Homology 2 (SH2). These domains could interact with other effector proteins such as GAP/Ras-GTP; C2/Annexin A6; SH2/downstream of tyrosine kinase (pTyr(Dok-1/-2) ), p190-RhoGAP (GAP protein for the Rho family of small GTPases), p200-RhoGAP ( GAP protein for the Rho family of small GTPases), SOCS3 (transcriptional suppressor of cytokine signaling ) and translation elongation factors (eEF1-A1/ eEF1-A2); SH3/G3BP (GAP SH3-binding protein), p14 (unknown p14-kDa protein), Aurora kinases, Capns1 (small calpain subunit-1) and p200-RhoGAP.
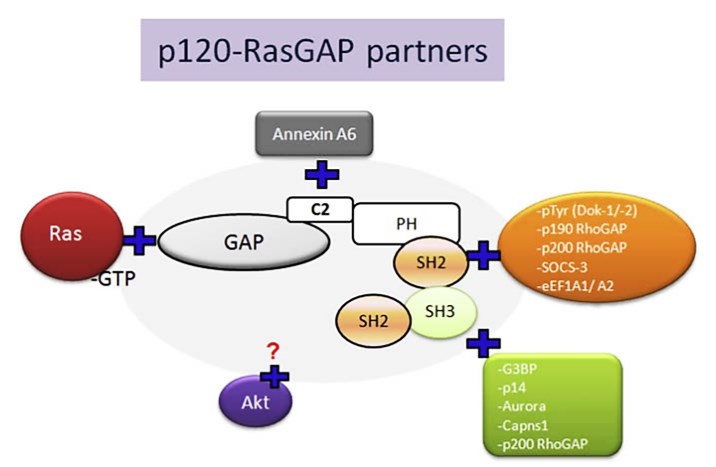 Figure 3. p120-RasGAP binding partners.
Figure 3. p120-RasGAP binding partners.
References
- Colicelli J. Human RAS superfamily proteins and related GTPases [J]. Sci Stke, 2004(250): RE13.
- Wennerberg, K. The Ras superfamily at a glance[J]. Journal of Cell Science, 2005, 118(5): 843-846.
- José B Pereira-Leal, Seabra M C. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily[J]. Journal of Molecular Biology, 2000, 301(4): 1077-1087.

