Apoptosis Intracellular
Creative BioMart Apoptosis Intracellular Product List
Immunology Background
Background
Intracellular signaling is fundamental to the regulation and execution of apoptosis, orchestrating the precise movement and localization of key proteins that mediate this process. Apoptosis is a highly regulated process involving intricate intracellular signaling pathways and molecular interactions. Intracellular kinases such as MAPKs and the PI3K/Akt pathway play a critical role in modulating apoptosis by affecting the stability and activity of pro- and anti-apoptotic factors. The Bcl-2 family of proteins, through its anti-apoptotic and pro-apoptotic members, regulates mitochondrial integrity and the release of apoptogenic factors. Caspases, the executioners of apoptosis, are critical for the execution of the death program by cleaving essential cellular substrates. The NFκB signaling pathway, with its complex network of subunits and interactions, serves as a key regulator of cell survival and apoptosis. Understanding these components and their interactions provides valuable insights into the mechanisms underlying apoptosis and holds potential for therapeutic intervention in diseases where apoptosis is dysregulated, such as cancer and autoimmune diseases.
Key Components of Apoptosis Intracellular Signaling
Apoptosis Intracellular Kinases
Intracellular kinases are central to the regulation of apoptosis, acting as signaling intermediates that integrate external stimuli and internal signals to modulate cell fate. Key kinases involved in apoptosis include mitogen-activated protein kinases (MAPKs) and the phosphoinositide 3-kinase (PI3K)/Akt pathway.
MAPKs
The MAPK pathway includes several families of kinases, including ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38 MAPK. Each of these MAPKs plays a distinct role in apoptosis:
- ERK: Typically associated with promoting cell survival and proliferation, ERK can also influence apoptosis under certain conditions. ERK activation often leads to the phosphorylation of various transcription factors and proteins involved in cell growth. However, prolonged ERK activation can result in cellular stress and apoptosis by upregulating pro-apoptotic factors and modulating the expression of anti-apoptotic proteins.
- JNK: JNK is strongly linked to the induction of apoptosis. It responds to cellular stress and inflammatory signals, leading to the phosphorylation of transcription factors like c-Jun. Activated JNK enhances the expression of pro-apoptotic genes and promotes apoptosis by upregulating proteins such as Bax and Puma, which facilitate mitochondrial outer membrane permeabilization.
- p38 MAPK: Similarly to JNK, p38 MAPK plays a role in the response to stress and inflammatory signals. p38 MAPK activation can result in apoptosis by modulating the expression of pro-apoptotic genes and influencing the stability of apoptosis regulators. Furthermore, it interacts with other signaling pathways to coordinate the apoptotic response.
PI3K/Akt Pathway
The PI3K/Akt pathway plays a pivotal role in the regulation of apoptosis. Upon activation by growth factors and other signals, PI3K (phosphoinositide 3-kinase) generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which recruits and activates Akt (also known as protein kinase B). Akt functions to promote cell survival through the following mechanisms:
Akt inhibits pro-apoptotic factors, including Bad and Bax, by phosphorylating them, thereby preventing their pro-apoptotic activities and stabilizing anti-apoptotic proteins, such as Bcl-2 and Bcl-xL. Akt activates the mechanistic target of rapamycin (mTOR), which plays a role in supporting cell growth and survival. mTOR signaling enhances protein synthesis and inhibits autophagy, contributing to cell survival. Moreover, Akt can modulate the formation of the apoptosome by influencing the activity of Apaf-1 (Apoptotic Protease Activating Factor-1), which in turn affects the activation of caspases.
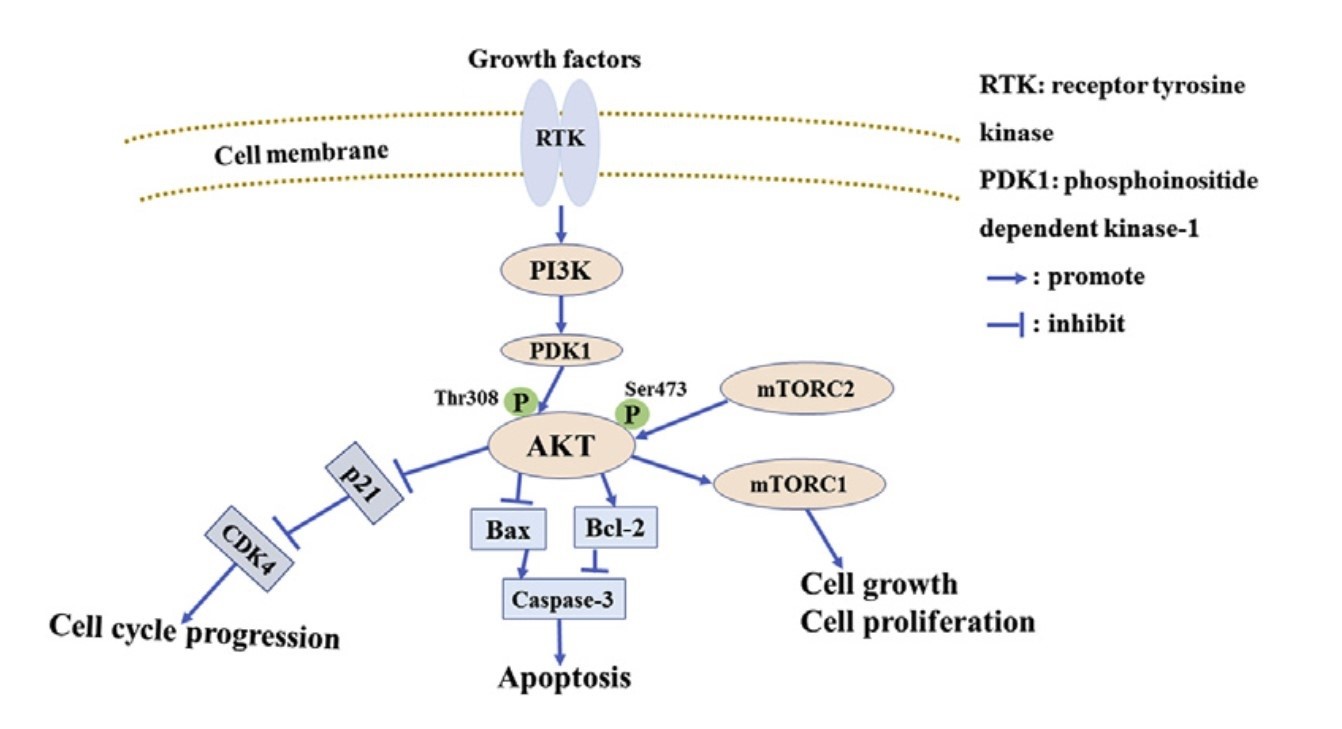 Fig. 1: PI3K/Akt pathway induces apoptosis (Wu et al., 2020).
Fig. 1: PI3K/Akt pathway induces apoptosis (Wu et al., 2020).Bcl-2 Family Proteins
The Bcl-2 family of proteins plays a crucial role in regulating mitochondrial outer membrane permeabilization (MOMP), a key event in the intrinsic apoptotic pathway. This family includes both pro-apoptotic and anti-apoptotic members that interact to determine cell fate.
Anti-Apoptotic Proteins
- Bcl-2: Bcl-2, one of the first identified members of this family, inhibits apoptosis by binding to and sequestering pro-apoptotic proteins. By stabilizing the mitochondrial membrane, Bcl-2 prevents the release of cytochrome c and other apoptogenic factors.
- Bcl-xL: Like Bcl-2, Bcl-xL protects cells from apoptosis by inhibiting pro-apoptotic Bcl-2 family members. It plays a significant role in maintaining the integrity of the mitochondrial membrane.
- Mcl-1: Mcl-1 is another anti-apoptotic protein that inhibits apoptosis by interacting with pro-apoptotic members. Its stability and expression are tightly regulated by various signaling pathways.
Pro-Apoptotic Proteins
- Bax and Bak: Bax and Bak are key pro-apoptotic members that promote MOMP. Upon activation, they undergo conformational changes, oligomerize, and insert into the mitochondrial membrane, leading to cytochrome c release and apoptosis.
- Bid: Bid is a BH3-only protein that is activated through cleavage by caspases. It translocates to the mitochondria and interacts with Bax and Bak, enhancing their pro-apoptotic activity.
- Puma and Noxa: Puma and Noxa are additional BH3-only proteins that promote apoptosis by binding to and neutralizing anti-apoptotic Bcl-2 family members, thereby facilitating Bax/Bak activation.
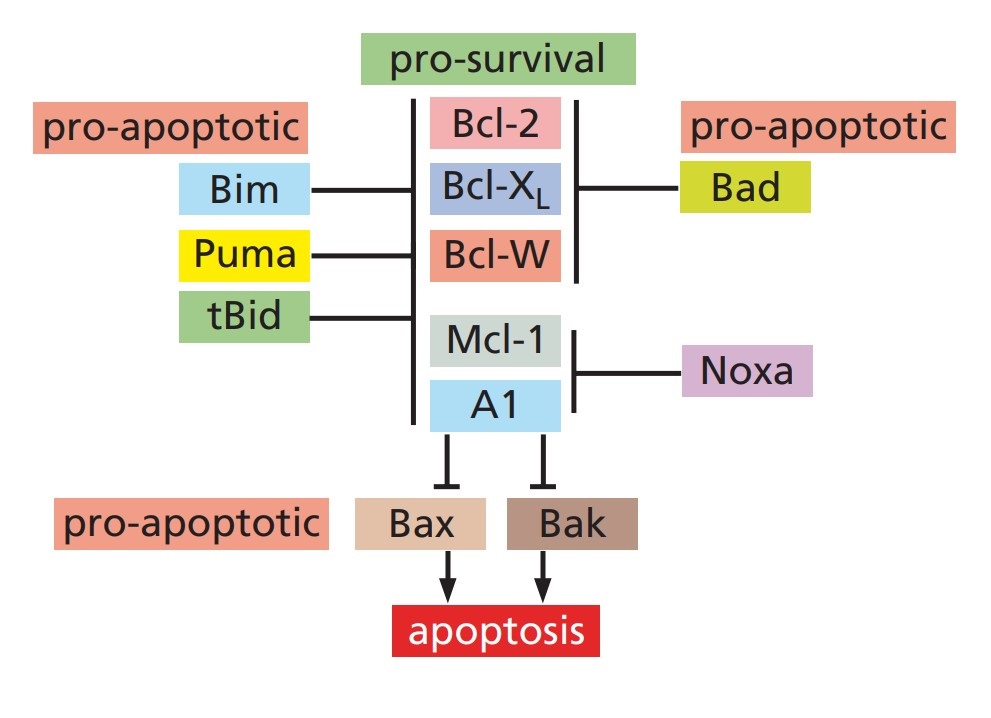 Fig. 2: Anti-apoptotic and pro-apoptotic Bcl-2 family proteins (The Biology of Cancer, Garland Science, 2nd Edition).
Fig. 2: Anti-apoptotic and pro-apoptotic Bcl-2 family proteins (The Biology of Cancer, Garland Science, 2nd Edition).Caspases
Caspases (cysteine-aspartic proteases) are essential effectors of apoptosis, responsible for executing the death program by cleaving specific substrates. They are synthesized as inactive pro-caspases and are activated in response to apoptotic signals.
Initiator Caspases
- Caspase-8: Caspase-8 is a key initiator caspase in the extrinsic apoptotic pathway, activated by death receptors such as Fas and TRAIL receptors. Upon activation, it cleaves and activates downstream effector caspases.
- Caspase-9: Caspase-9 is an initiator caspase in the intrinsic apoptotic pathway, activated by the apoptosome complex formed by cytochrome c, Apaf-1, and ATP. Caspase-9 activates downstream caspases, leading to apoptosis.
Effector Caspases
- Caspase-3: Caspase-3 is one of the major executioner caspases, responsible for the cleavage of numerous cellular substrates, including structural proteins and enzymes, leading to the morphological and biochemical changes associated with apoptosis.
- Caspase-6: Caspase-6 is a crucial cysteine protease involved in the execution phase of apoptosis. It is activated in response to apoptotic signals and is responsible for cleaving specific substrates that lead to cellular dismantling and death.
- Caspase-7: Similar to caspase-3 and caspase-6, caspase-7 also plays a role in executing apoptosis by cleaving substrates involved in maintaining cell structure and function.
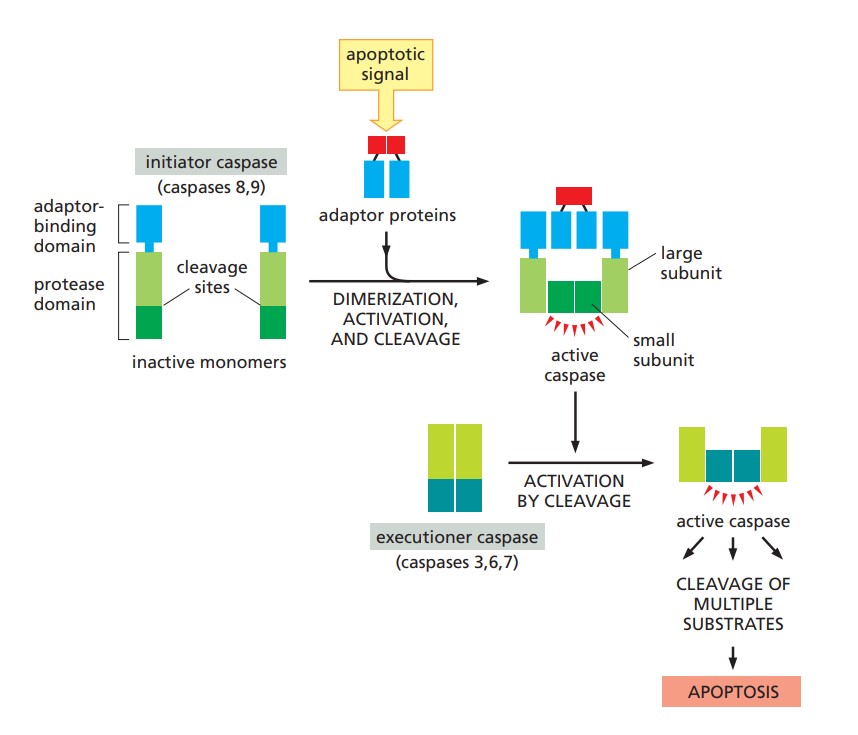 Fig. 3: Caspase activation during apoptosis (Molecular Biology of the Cell, Garland Science, 6th Edition).
Fig. 3: Caspase activation during apoptosis (Molecular Biology of the Cell, Garland Science, 6th Edition).NF-κB Pathway
The NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) signaling pathway is a critical regulator of cell survival and apoptosis. It consists of several subunits, including p65 (RelA), p50, p52, RelB, and Bcl-3, which form various dimers and complexes.
Canonical NF-κB Pathway
The canonical NF-κB pathway is typically activated by pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNFα) and interleukin-1 (IL-1). These signals lead to activation of the IKK (IκB kinase) complex, which phosphorylates IκB proteins, resulting in their degradation and release of NF-κB dimers such as p65. NF-κB generally promotes cell survival by inducing anti-apoptotic genes such as Bcl-2, Bcl-xL, and inhibitor of apoptosis proteins (IAPs). This anti-apoptotic activity helps cells resist stress and inflammation.
Non-Canonical NF-κB Pathway
The non-canonical NF-κB pathway is activated by signals such as those from lymphotoxin β receptor and CD40. This pathway involves the processing of p100 to p52 and the formation of p52 dimers. The alternative NF-κB pathway regulates gene expression in immune cells and contributes to cell survival. It also influences lymphoid organ development and the maintenance of immune homeostasis.
Case Study
Case 1: Falahi, F.; et al. Caspase-9 suppresses metastatic behavior of MDA-MB-231 cells in an adaptive organoid model. Scientific Reports, 2024; 14(1), 15116.
Caspase 9, a cysteine-aspartate protease traditionally associated with intrinsic apoptosis, has recently been shown to have non-apoptotic roles, including influencing cell migration. In this study, the researchers investigated the effect of caspase 9 on the migration and invasion behavior of MDA-MB-231, a triple-negative breast cancer (TNBC) cell line known for its metastatic properties. They established a stable cell line expressing an inducible caspase-9 (iC9) in MDA MB 231 and evaluated its metastatic behavior using both monolayer and 3D organotypic models in co-culture with human foreskin fibroblasts (HFF). The results showed that caspase-9 had an inhibitory effect on migration and invasion in both models, monolayer culture and organotypic model.
Inducible caspase-9 is a genetically engineered form of human caspase-9 characterized by the absence of the caspase activation and recruitment domain (CARD). Consequently, its dimerization and activation require a chemical dimerization inducer such as AP20187 or AP1903, which have been shown to be safe in human cells. In parallel, the caspase-9-mediated suppression of metastasis was compared with the anti-metastatic agent Panitumumab (Pan). To evaluate the influence of the iC9/AP20187 system on cell migration within a 2D microenvironment, a scratch assay was performed. The result showed that the migration rate of mock cells was higher than that of all iC9 transduced cells in all groups. The results also showed a significant increase in the inhibition of the migration rate of AP20187 treated iC9 transduced cells compared to the untreated group. Pan treatment also increased, although to a lesser extent. Notably, the combination of AP20187 and Pan resulted in the most significant increase in migration inhibition.
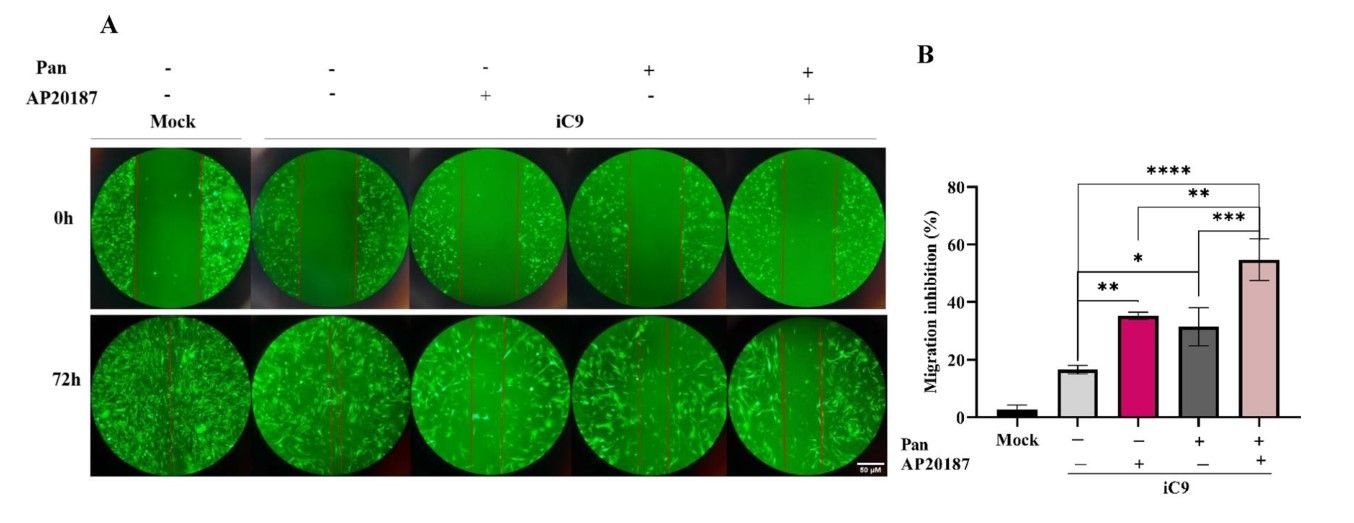 Fig. 4: The iC9/AP20187 system reduces the migration and invasion of MDA-MB-231 cells. (A) To capture images of migrated cells in the monolayer under different media conditions with or without AP20187 and Pan, imaging was employed over a 72-h time course (magnification 10×, Scale Bar; 50 µm). (B) The migration inhibition percentage in wound healing assay was quantified. The data are expressed as mean ± SD of three independent replicates with significant differences denoted as *p < 0.05, **p < 0.01, and ***p < 0.001. The significant differences of mock group compared to iC9 group was (p-value = 0.025) and with other groups was less than 0.001.
Fig. 4: The iC9/AP20187 system reduces the migration and invasion of MDA-MB-231 cells. (A) To capture images of migrated cells in the monolayer under different media conditions with or without AP20187 and Pan, imaging was employed over a 72-h time course (magnification 10×, Scale Bar; 50 µm). (B) The migration inhibition percentage in wound healing assay was quantified. The data are expressed as mean ± SD of three independent replicates with significant differences denoted as *p < 0.05, **p < 0.01, and ***p < 0.001. The significant differences of mock group compared to iC9 group was (p-value = 0.025) and with other groups was less than 0.001.Case 2: Dong, L.; Vaux, D. L. Glucocorticoids can induce BIM to trigger apoptosis in the absence of BAX and BAK1. Cell Death & Disease, 2020; 11(6), 1–15.
BAX and BAK1 are generally thought to be essential for cells to undergo apoptosis by the "intrinsic"; or "mitochondrial"; pathway. In addition, BH3-only proteins such as BIM and BAD are thought to require the presence of BAX or BAK1 to kill cells. However, in this study, Dong and Vaux show that glucocorticoids can induce BIM to induce apoptosis in the absence of BAX and BAK1.
Using CRISPR/Cas9, the Bax-/- Bak1-/- WEHI7 cells and other knockout cell lines were generated and treated with dexamethasone (Dex), a fluorinated glucocorticoid drug. To determine which caspases were processed when the Bax-/- Bak1-/- WEHI7 cells were treated with Dex, lysates were analyzed by Western blot using antibodies specific for cleaved caspases. At 24 h, Dex induced cleavage and activation of both caspase 9 and caspase 3 in wild-type WEHI7 cells. Although no activated caspases could be detected at this time point in Bax-/- Bak1-/- WEHI7 cells, both caspase 9 and caspase 3 were cleaved after 6 days of exposure to Dex. Similarly, cleaved caspases were detected in the Bax-/- Bak1-/- p53-/- T lymphoma line after 2 days of treatment with Dex.
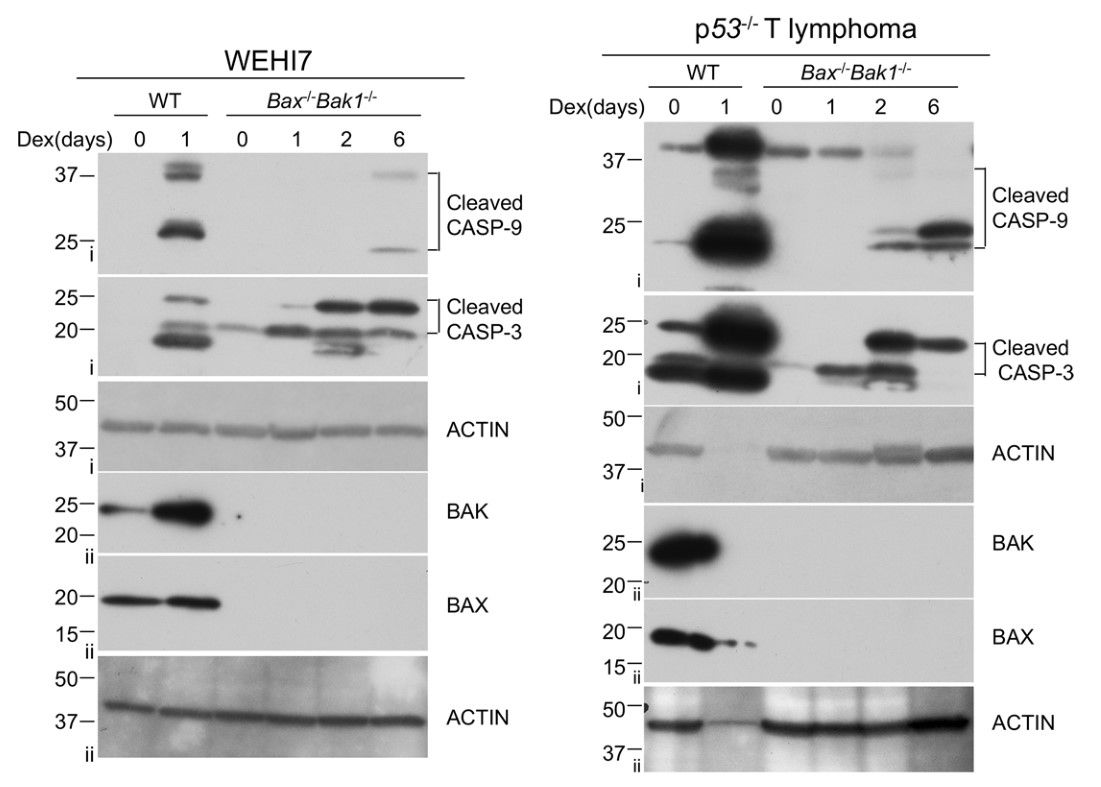 Fig. 5: Dexamethasone can induce caspase activation in the absence of BAX and BAK1. a WEHI7 cells (left panel) and p53-/- lymphoma cells (right panel) from each genotype (Bax+/+ Bak1+/+ and Bax-/- Bak1-/-) were treated with 1 µM Dex for indicated times. Cell lysates were analyzed by western blot with antibodies to cleaved Caspase-3, cleaved Caspase-9, BAK, BAX, and ACTIN. Data show results of one of two independent experiments. Roman numerals to the left of blots (i–ii) indicate the membrane probed.
Fig. 5: Dexamethasone can induce caspase activation in the absence of BAX and BAK1. a WEHI7 cells (left panel) and p53-/- lymphoma cells (right panel) from each genotype (Bax+/+ Bak1+/+ and Bax-/- Bak1-/-) were treated with 1 µM Dex for indicated times. Cell lysates were analyzed by western blot with antibodies to cleaved Caspase-3, cleaved Caspase-9, BAK, BAX, and ACTIN. Data show results of one of two independent experiments. Roman numerals to the left of blots (i–ii) indicate the membrane probed.References
- Molecular biology of the cell (6th ed). (2015). Garland Science, Taylor and Francis group.
- The biology of cancer (2nd ed). (2014). Garland Science, Taylor and Francis group. Weinberg, R. A.
- Dong, L., & Vaux, D. L. (2020). Glucocorticoids can induce BIM to trigger apoptosis in the absence of BAX and BAK1. Cell Death & Disease, 11(6), 1–15.
- Falahi, F., Akbari-Birgani, S., Mortazavi, Y., & Johari, B. (2024). Caspase-9 suppresses metastatic behavior of MDA-MB-231 cells in an adaptive organoid model. Scientific Reports, 14(1), 15116.
- Guo, Y.-J., Pan, W.-W., Liu, S.-B., Shen, Z.-F., Xu, Y., & Hu, L.-L. (2020). ERK/MAPK signalling pathway and tumorigenesis (Review). Experimental and Therapeutic Medicine, 19(3), 1997–2007.
- Wu, Y., Ma, J., Sun, Y., Tang, M., & Kong, L. (2020). Effect and mechanism of PI3K/AKT/mTOR signaling pathway in the apoptosis of GC-1 cells induced by nickel nanoparticles. Chemosphere, 255, 126913.

