Bcl-2 Family
Related Symbol Search List
- BAG2
- BAG3
- BAK1
- BAX
- BCKDK
- BCL10
- Bcl-2
- Bcl2A1
- BCL2L1
- BCL2L11
- BCL2L12
- BCL2L2
- BID
- BIK
- BNIP3
- BNIP3L
- BOK
- DYNLL1
- HRK
- Mcl-1
- MASP1
- MOAP1
Immunology Background
The B cell lymphoma 2 (BCL‑2) gene family encodes more than 20 proteins that have a central role in regulating programmed cell death by controlling pro-apoptotic and anti-apoptotic intracellular signals, and are fundamental to the balance between cell survival and death [1-3].
BCL2 was initially discovered as part of the t(14;18) chromosomal translocation, which occurs in patients with follicular lymphoma and diffuse large B cell lymphoma (DLBCL), and leads to elevated BCL2 transcription [4-5]. Although originally believed to act as a classical growth-driving oncogene, it was later shown that BCL2 instead promotes malignant cell survival by attenuating apoptosis [6-7]. Members of the BCL‑2 family are grouped together, as they contain up to four conserved BCL‑2 homology (BH) regions. The multi-region (BH1–4) anti-apoptotic proteins BCL‑2, BCL‑XL (also known as BCL‑2L1), BCL‑W (also known as BCL‑2L2), myeloid cell leukaemia 1 (MCL1) and A1 (also known as BCL‑2A1) antagonize pro-apoptotic BH3‑only proteins, and they inhibit the essential apoptosis effectors BCL‑2 antagonist killer [7] (BAK) and BCL‑2‑associated X protein (BAX) [8]. BCL2 dysregulation results in the overexpression of the anti-apoptotic protein BCL‑2, which alters the balance between pro-apoptotic and anti-apoptotic members of the BCL‑2 family [9].
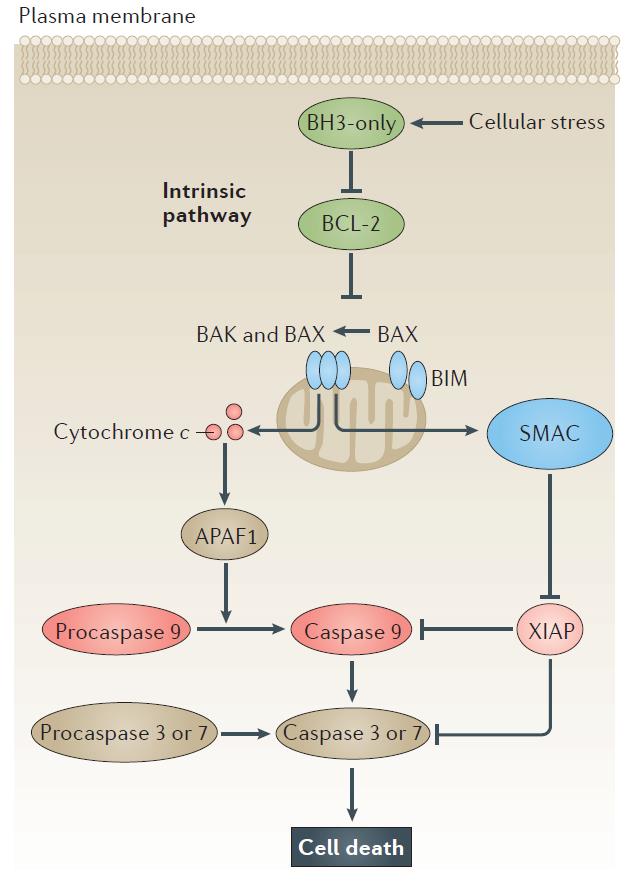 Fig1. The intrinsic apoptosis pathway. [17]
Fig1. The intrinsic apoptosis pathway. [17]
Programmed cell death, or apoptosis, is critical to both development and maintenance of tissues, as well as to the destruction of the cell when injured. The BCL-2 family proteins and the inhibitors of apoptosis proteins (AIPs) are the major regulators of the apoptotic process, whereas caspases, a family of cysteine proteases, are the major executioners of this process [16]. Following activation of the intrinsic pathway by cellular stress, pro-apoptotic BCL‑2 homology 3 (BH3) ‑only proteins inhibit the anti-apoptotic proteins B cell lymphoma 2 (BCL‑2), BCL‑XL, BCL‑W and myeloid cell leukaemia 1 (MCL1). The subsequent activation and oligomerization of the pro-apoptotic proteins BCL‑2 antagonist killer 1 (BAK) and BCL‑2‑associated X protein (BAX) results in mitochondrial outer membrane permeabilization (MOMP). This results in the release of cytochrome c and second mitochondria-derived activator of caspase (SMAC; also known as DIABLO) from the mitochondria. Cytochrome c forms a complex with procaspase 9 and apoptosis protease-activating factor 1 (APAF1), which leads to the activation of caspase 9. Caspase 9 then activates procaspase 3 and procaspase 7, resulting in cell death. Inhibition of this process by anti-apoptotic BCL‑2 proteins occurs via sequestration of pro-apoptotic proteins through binding to their BH3 motifs. BIM, BCL‑2‑interacting mediator of cell death; XIAP, X‑linked inhibitor of apoptosis. [15, 17]
Nowadays, studies showed that the inhibition of apoptosis related Bcl-2 family proteins is thought to lead to chemoresistance [10] and has been identified in many cancers, including haematological malignancies [11] (such as multiple myeloma, chronic lymphocytic leukaemia (CLL), acute lymphocytic leukaemia (ALL), acute myeloid leukaemia (AML), myelodysplastic syndrome and myeloproliferative neoplasms) and solid tumours (such as breast cancer [12], lung cancer [13], melanoma [14] and mesothelioma [15]. Therefore, as BCL‑2‑mediated resistance to intrinsic apoptosis is a hallmark of malignancy, targeting the anti-apoptotic BCL‑2 proteins is an attractive therapeutic strategy in cancer.
Ref:
- Shamas-Din, A., Brahmbhatt, H., Leber, B. & Andrews, D. W. BH3‑only proteins: orchestrators of apoptosis. Biochim. Biophys. Acta 1813, 508–520 (2011).
- Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL‑2 family reunion. Mol. Cell 37, 299–310 (2010).
- Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL‑2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
- Tsujimoto, Y., Finger, L. R., Yunis, J., Nowell, P. C. & Croce, C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 (1984).
- Tsujimoto, Y. et al. Molecular cloning of the chromosomal breakpoint of B‑cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science 224, 1403–1406 (1984).
- Vaux, D. L., Cory, S. & Adams, J. M. Bcl‑2 gene promotes haemopoietic cell survival and cooperates with c‑myc to immortalize pre‑B cells. Nature 335, 440–442 (1988).
- McDonnell, T. J. et al. bcl‑2‑immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57, 79–88 (1989).
- O’Neill, K. L., Huang, K., Zhang, J., Chen, Y. & Luo, X. Inactivation of prosurvival Bcl‑2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 30, 973–988 (2016).
- Kang, M. H. & Reynolds, C. P. Bcl‑2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 15, 1126–1132 (2009).
- Amundson, S. A. et al. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60, 6101–6110 (2000).
- Kitada, S., Pedersen, I. M., Schimmer, A. D. & Reed, J. C. Dysregulation of apoptosis genes in hematopoietic malignancies. Oncogene 21, 3459–3474 (2002).
- Vaillant, F. et al. Targeting BCL‑2 with the BH3 mimetic ABT‑199 in estrogen receptor-positive breast cancer. Cancer Cell 24, 120–129 (2013).
- Gandhi, L. et al. Phase I study of navitoclax (ABT‑263), a novel Bcl‑2 family inhibitor, in patients with small cell lung cancer and other solid tumors. J. Clin. Oncol. 29, 909–916 (2011).
- Bedikian, A. Y. et al. Bcl‑2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J. Clin. Oncol. 24, 4738–4745 (2006).
- Cao, X. X., Mohuiddin, I., Ece, F., McConkey, D. J. & Smythe, W. R. Histone deacetylase inhibitor downregulation of bcl‑xl gene expression leads to apoptotic cell death in mesothelioma. Am. J. Respir. Cell Mol. Biol. 25, 562–568 (2001).
- J.C. Martinou, R.J. Youle, Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics, Developmental cell, 21 (2011) 92-101.
- Avi Ashkenazi1, Wayne J. Fairbrother1, Joel D. Leverson2 and Andrew J. Souers. From basic apoptosis discoveries to advanced selective BCL‑2 family inhibitors. NATURE REVIEWS. DRUG DISCOVERY 2017

