MHC Class II
Related Symbol Search List
- LILRB1
- ILT4
- LILRB3
- LILRA1
- CD4
- ERAP1
- ERAP2
- FCGRT
- HLA-A
- HLA-C
- HLA-E
- KLRC3
- LILRB5
- MR1
- TAOK3
- TAPBP
- TAPBPL
Immunology Background
Background
About MHC Class II
MHC class II molecules are key components of the immune system, responsible for presenting peptide antigens derived from extracellular pathogens to helper CD4+ T cells. This presentation of antigens plays a critical role in the activation and regulation of the adaptive immune response.
Structurally, MHC class II molecules consist of two main chains: an α chain and a β chain. These chains are encoded by genes within the MHC locus in humans, known as the Human Leukocyte Antigen (HLA) complex. Each chain comprises extracellular domains, a transmembrane region, and a short cytoplasmic tail.
The extracellular domains of both the α and β chains are composed of two regions: the α1 and α2 domains in the α chain, and the β1 and β2 domains in the β chain. These domains form a peptide-binding groove, where antigenic peptides bind. The α1 and β1 domains are primarily responsible for peptide binding, while the α2 and β2 domains contribute to the overall stability and structure of the molecule.
In addition to the α and β chains, MHC class II molecules also associate with a molecule called the invariant chain (Ii). The Ii molecule is synthesized in the endoplasmic reticulum and helps to guide the MHC class II molecules through the intracellular pathway. It also serves to block the binding of endogenous peptides to the peptide-binding groove during the assembly process.
The presentation of antigens by MHC class II molecules is crucial for the coordination of immune responses. By presenting antigens to CD4+ T cells, MHC class II molecules facilitate the activation of B cells, cytotoxic T cells, and other effector cells. This activation leads to the production of specific antibodies, the elimination of pathogens, and the establishment of immunological memory.
The polymorphic nature of MHC class II molecules allows for an extensive range of peptide antigens to be presented, ensuring diversity in the immune response and the ability to recognize a wide variety of pathogens.
Overall, MHC class II molecules and their role in antigen presentation are essential for the adaptive immune system to mount effective immune responses against extracellular pathogens, contributing to the overall defense and protection of the organism.
Relationship Between MHC Class II and Antigen Presentation
MHC class II molecules play a critical role in antigen presentation, specifically in the presentation of peptide antigens derived from extracellular pathogens. Antigen presentation refers to the process by which antigens are displayed on the cell surface for recognition by immune cells, such as CD4+ T cells.
The process of MHC class II-mediated antigen presentation involves several steps:
- Antigen Internalization: Antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B cells, internalize extracellular pathogens or their components through processes such as phagocytosis, endocytosis, or receptor-mediated uptake.
- Antigen Processing: Once internalized, the pathogens are degraded within specialized compartments called endosomes and lysosomes. These compartments contain enzymes that break down the pathogens into smaller fragments, including peptide antigens.
- MHC Class II Synthesis and Assembly: Within the endocytic compartments, a specialized compartment called the MHC class II compartment (MIIC) serves as the site where MHC class II molecules are synthesized and assembled. The α and β chains of MHC class II molecules combine to form a heterodimeric structure.
- Invariant Chain (Ii) Binding: During synthesis, MHC class II molecules associate with a molecule called the invariant chain (Ii). The Ii molecule acts as a chaperone, guiding the MHC class II molecules through the intracellular pathway and preventing premature binding of endogenous peptides.
- Invariant Chain Processing: Within the MIIC, the invariant chain is proteolytically cleaved, leaving a small fragment called the class II-associated invariant chain peptide (CLIP) bound to the peptide-binding groove of the MHC class II molecule. CLIP serves to block the binding of endogenous peptides.
- Antigen Loading: Antigenic peptides derived from the degraded pathogens replace CLIP in the peptide-binding groove of MHC class II molecules. This process is facilitated by specialized molecules known as HLA-DM in humans (HLA-DO in certain contexts), which assist in the removal of CLIP and the loading of antigenic peptides.
- Cell Surface Expression: Once loaded with antigenic peptides, the MHC class II-antigen complex is transported to the cell surface, where it is presented to CD4+ T cells for recognition.
- CD4+ T Cell Activation: CD4+ T cells express a T cell receptor (TCR) on their surface, which recognizes specific peptide antigens bound to MHC class II molecules. When the TCR interacts with the antigenic peptide-MHC class II complex, it triggers signaling events that lead to the activation of the CD4+ T cell. This activation initiates a cascade of immune responses, including the production of cytokines and the activation of other immune cells.
By presenting antigenic peptides to CD4+ T cells, MHC class II molecules play a crucial role in initiating and regulating adaptive immune responses. The recognition of antigenic peptides by CD4+ T cells leads to the activation of various immune effector mechanisms, such as the production of antibodies by B cells, the activation of cytotoxic T cells, and the modulation of immune responses to control pathogens.
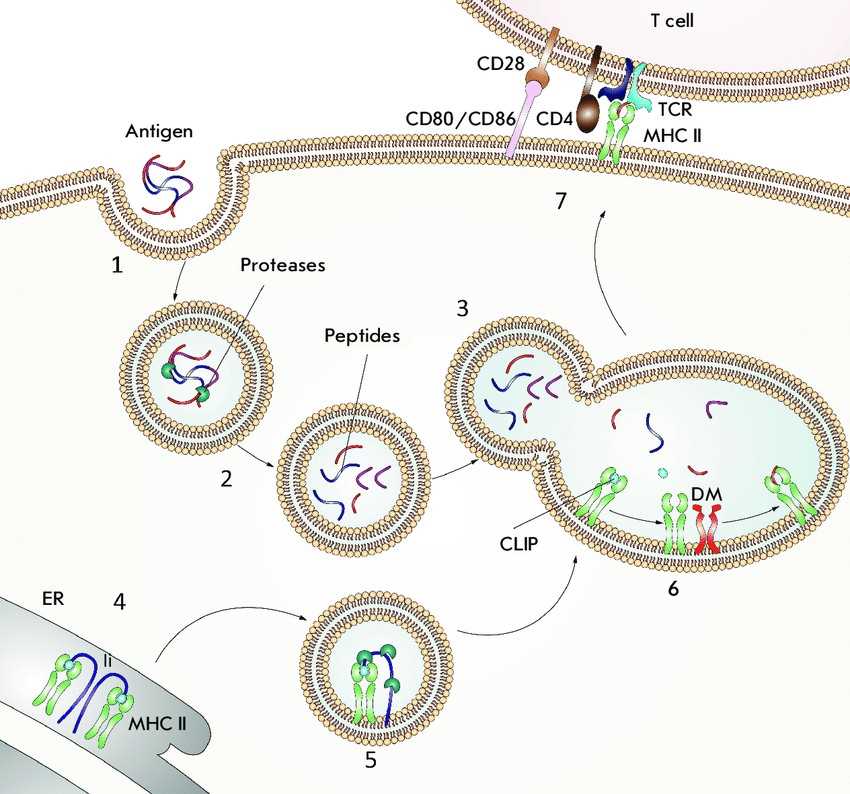 Fig.1 Antigen presentation by MHC II molecules. (Zakharova MY, et al., 2019)
Fig.1 Antigen presentation by MHC II molecules. (Zakharova MY, et al., 2019)(1) An antigen enters intracellular vesicles. (2) Acidification of vesicles activates proteases that hydrolyze the antigen into peptide fragments. (3) Vesicles containing the peptide fragments merge with vesicles containing MHC II molecules (green). (4) The invariant chain (Ii) (violet) binds to the newly synthesized MHC II molecules and partially occupies the peptide-binding groove. (5) The invariant chain undergoes proteolytic degradation; as a result, the CLIP peptide (blue) remains bound in the groove. (6) DM (orange) binds to the MHC II molecules and catalyzes the peptide exchange. (7) The MHC II molecules, loaded with an antigenic peptide (red), are transported to the cell surface where they can be recognized by a CD4 + T cell receptor TCR (cyan blue). The CD4 co-receptor molecule (brown) present on T cells also binds to the MHC II molecules. For T-cell activation to occur, the CD80 or CD86 co-stimulating molecules (pink) expressed on the antigen-presenting cell must bind to the CD28 co-stimulating molecule (beige) expressed on the T cells.
Molecules of zMHC Class II
The following are some of the key molecules associated with MHC class II:
| Key molecule types | Functions |
|---|---|
| Invariant Chain (Ii) | The invariant chain is a chaperone protein that associates with MHC class II molecules early in their biosynthesis. It helps in the proper folding and assembly of MHC class II molecules and prevents premature binding of endogenous peptides. The invariant chain is proteolytically cleaved within the endocytic compartments, leaving a small fragment called class II-associated invariant chain peptide (CLIP) bound to the peptide-binding groove of MHC class II molecules. |
| HLA-DM and HLA-DO | These molecules are involved in the exchange of CLIP with antigenic peptides within the MHC class II peptide-binding groove. HLA-DM facilitates the release of CLIP and promotes the binding of antigenic peptides, while HLA-DO acts as a modulator of HLA-DM activity. Together, HLA-DM and HLA-DO contribute to the efficient loading of antigenic peptides onto MHC class II molecules. |
| Endosomal Proteases | Within the endocytic compartments, various proteases play a crucial role in the degradation of internalized pathogens and the generation of antigenic peptides. These proteases, such as cathepsins, cleave the pathogens into smaller fragments, including the antigenic peptides that will be loaded onto MHC class II molecules. |
| TAP (Transporter Associated with Antigen Processing) | TAP is a heterodimeric transporter protein located in the endoplasmic reticulum (ER). It transports antigenic peptides from the cytosol into the ER, where they can be further processed and loaded onto MHC class II molecules. While TAP primarily functions in the context of MHC class I antigen presentation, it can also contribute to the presentation of certain peptides by MHC class II molecules. |
| Peptide Loading Complex (PLC) | The PLC is a multi-protein complex that is involved in the loading of antigenic peptides onto MHC class II molecules within the MIIC. It includes components such as MHC class II molecules themselves, the invariant chain, HLA-DM, HLA-DO, and other accessory molecules. The PLC ensures the proper coordination and regulation of peptide loading onto MHC class II molecules. |
Regulation of MHC Class II in Disease Development
MHC class II molecules play a significant role in disease development, particularly in autoimmune and infectious diseases. Recent advances have shed light on their involvement and provided insights into disease mechanisms. Here are examples of how MHC class II molecules contribute to autoimmune and infectious diseases:
Autoimmune Diseases
- Rheumatoid Arthritis (RA): MHC class II molecules, such as HLA-DRB1 alleles, are strongly associated with the development of RA. They present self-antigens to CD4+ T cells, initiating an autoimmune response that leads to chronic inflammation and joint damage.
- Systemic Lupus Erythematosus (SLE): MHC class II molecules, particularly HLA-DR and HLA-DQ, are implicated in SLE. They present self-antigens, including nucleic acid-derived antigens, to CD4+ T cells, resulting in the production of autoantibodies and tissue damage.
- Multiple Sclerosis (MS): MHC class II molecules, such as HLA-DRB1*15:01, are strongly associated with an increased risk of developing MS. They present myelin antigens to CD4+ T cells, triggering an autoimmune response that leads to inflammation and demyelination in the central nervous system.
Infectious Diseases
- HIV/AIDS: MHC class II molecules play a role in the immune response against HIV infection. They present viral antigens to CD4+ T helper cells, which are the primary targets of HIV. The interaction between viral proteins and MHC class II molecules influences the course of infection and the effectiveness of immune responses.
- Tuberculosis (TB): MHC class II molecules are essential for presenting Mycobacterium tuberculosis antigens to CD4+ T cells. The activation of T cells through MHC class II antigen presentation is crucial for controlling TB infection. Genetic variations in MHC class II genes can affect susceptibility to TB and the development of protective immune responses.
- Malaria: MHC class II molecules are involved in the immune response against Plasmodium falciparum, the parasite responsible for severe malaria. They present parasite-derived antigens to CD4+ T cells, promoting the activation of specific immune responses. Polymorphisms in MHC class II genes influence the susceptibility to severe malaria and the outcome of infection.
Advances in Understanding
Recent advances in autoimmune and infectious diseases have provided insights into the role of MHC class II molecules:
- Genetic Studies: Genome-wide association studies (GWAS) have identified specific MHC class II alleles associated with increased susceptibility or protection in autoimmune diseases, highlighting the importance of MHC class II in disease development.
- Epitope Mapping: Advances in epitope mapping have enabled the identification of disease-specific antigenic peptides presented by MHC class II molecules. This knowledge facilitates the development of targeted therapies and vaccines.
- Immunotherapies: Immunotherapeutic strategies, such as immune checkpoint inhibitors and antigen-specific immunotherapies, are being explored to modulate MHC class II antigen presentation and CD4+ T cell responses in autoimmune diseases and infectious diseases.
- Precision Medicine: The understanding of MHC class II genetics and antigen presentation has paved the way for personalized medicine approaches, where treatment strategies can be tailored based on an individual's MHC class II profile and antigenic repertoire.
In summary, MHC class II molecules play a crucial role in the development of autoimmune and infectious diseases. Advances in understanding their involvement have opened new avenues for diagnosis, treatment, and prevention of these diseases.
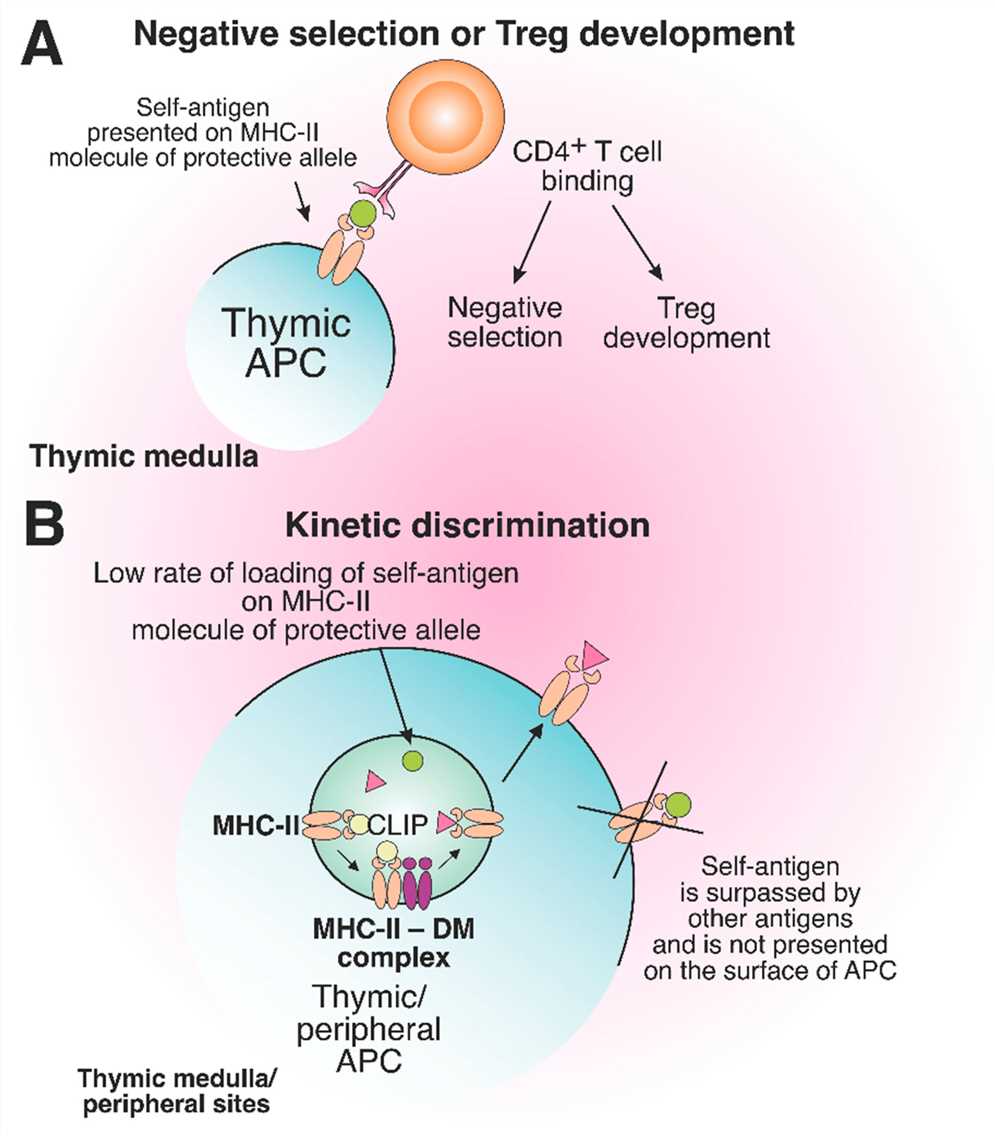 Fig.2 The role of protective MHC-II molecules in the suppression of autoreactive T cell development. (Ishina IA, et al., 2023)
Fig.2 The role of protective MHC-II molecules in the suppression of autoreactive T cell development. (Ishina IA, et al., 2023)Case Study
Case 1: Evans AM, Salnikov M, Tessier TM, Mymryk JS. Reduced MHC Class I and II Expression in HPV−Negative vs. HPV−Positive Cervical Cancers. Cells. 2022; 11(23):3911.
The MHC class II α- and β-chains are synthesized in the endoplasmic reticulum (ER), where they form a trimeric complex with a membrane glycoprotein called the invariant chain (Ii; encoded by CD74) to prevent premature loading with endogenously derived peptides. The cytoplasmic region of Ii directs the MHC class II complex through the Golgi and trans-Golgi network to early endosomes. These early endosomes contain cathepsins that proteolytically cleave Ii, leaving a class II-associated Ii peptide in the binding groove (CLIP). Similar to the genes encoding the MHC class II α- and β-chains, CD74 was expressed at remarkably high levels and was significantly upregulated in both α7 and α9 HPV+ CC compared to HPV− CC. This finding suggests higher activity of the antigen presentation pathway in HPV+ CC.
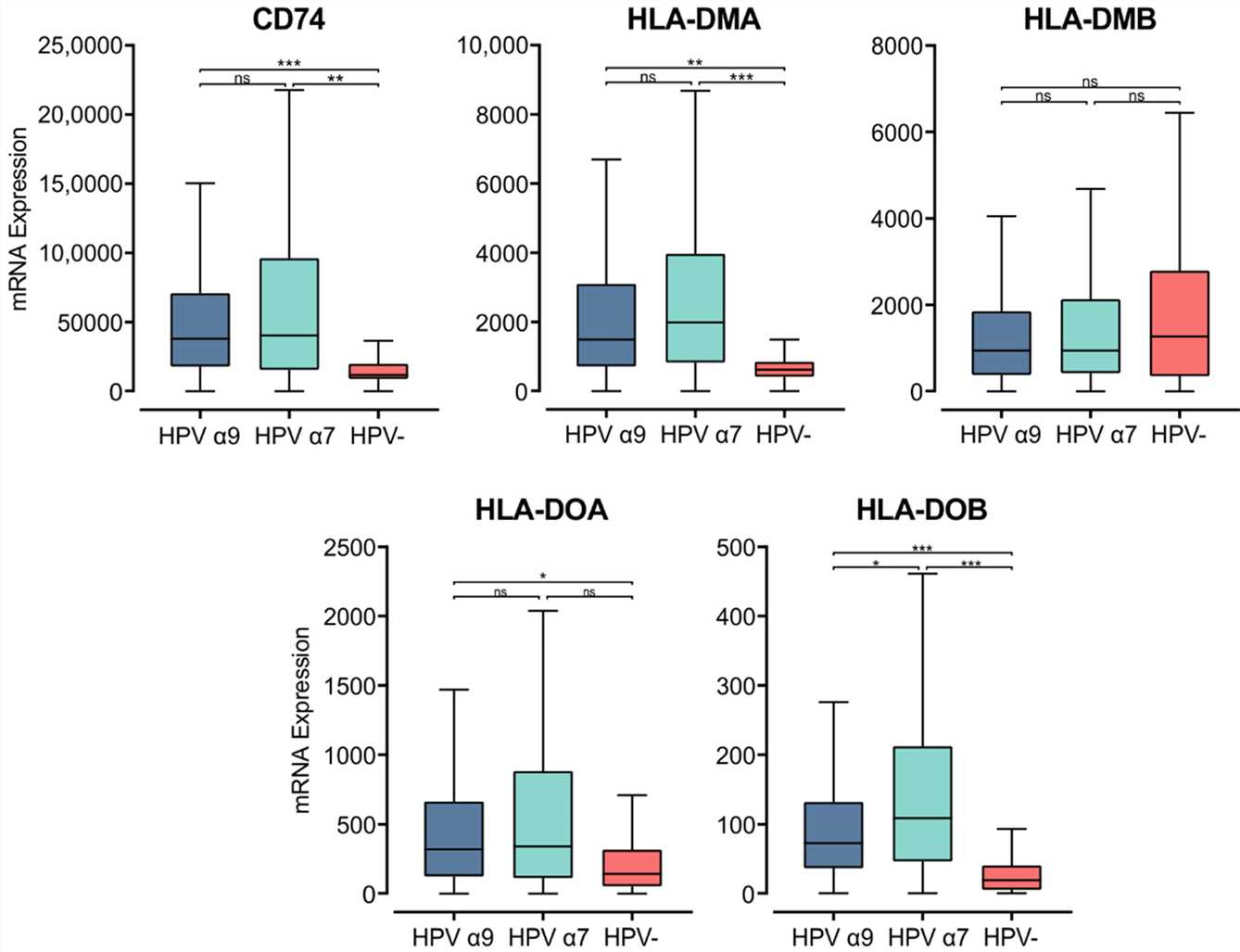 Fig.1 Expression of invariant chain (CD74) and MHC class II-like genes in CC stratified by HPV+ (α9 or α7) and HPV− status.
Fig.1 Expression of invariant chain (CD74) and MHC class II-like genes in CC stratified by HPV+ (α9 or α7) and HPV− status.Case 2: Chen X, Jensen PE. MHC class II antigen presentation and immunological abnormalities due to deficiency of MHC class II and its associated genes. Exp Mol Pathol. 2008;85(1):40-44.
The author team has developed a flow cytometry test to detect the expression of DM, DO and Ii in B cells. The flow cytometry assay combines cell surface and intracellular staining with specific monoclonal antibodies to unambiguously identify the expression of DM, DO, and Ii in peripheral blood B cells and can be readily employed in a clinical laboratory setting to facilitate screening for Ii, DM, or DO deficiency in patients with CVID.
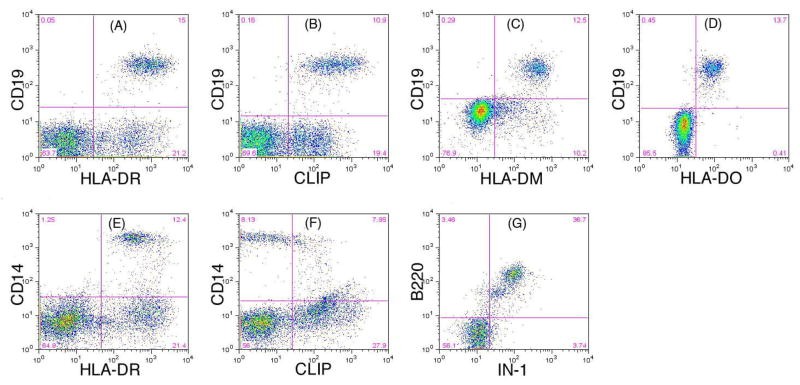 Fig.2 Identification of expression of HLA-DR, CLIP, DM, DO and Ii by flow cytometry.
Fig.2 Identification of expression of HLA-DR, CLIP, DM, DO and Ii by flow cytometry.References
- Zakharova MY, Belyanina TA, Sokolov AV, Kiselev IS, Mamedov AE. The Contribution of Major Histocompatibility Complex Class II Genes to an Association with Autoimmune Diseases. Acta Naturae. 2019;11(4):4-12.
- Ishina IA, Zakharova MY, Kurbatskaia IN, Mamedov AE, Belogurov AA Jr., Gabibov AG. MHC Class II Presentation in Autoimmunity. Cells. 2023; 12(2):314.
- Poluektov YO, Kim A, Sadegh-Nasseri S. HLA-DO and Its Role in MHC Class II Antigen Presentation. Front Immunol. 2013;4:260.
- Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023;20(9):604-623.

