Host Cell Protein Mitigation
Host Cell Proteins (HCPs) are residual impurities that can co-purify with recombinant therapeutic proteins and compromise drug safety, efficacy, and regulatory approval. Creative BioMart provides comprehensive Host Cell Protein Mitigation Services, offering expert-driven, customized strategies to identify, quantify, and reduce HCPs during biotherapeutic development and manufacturing. Utilizing a wide array of orthogonal analytical techniques—such as HCP ELISAs, LC-MS/MS, and electrophoretic methods—we ensure a thorough and compliant HCP profile. From method development and validation to interpretation and process optimization, we deliver an end-to-end solution to minimize HCP risk and accelerate your drug’s path to market.
Background: Understanding Host Cell Protein Contamination in Biologics
Recombinant therapeutic proteins produced in microbial or mammalian systems inevitably carry residual host-derived proteins, known as Host Cell Proteins (HCPs). These impurities can originate at any stage of the upstream or downstream process and are particularly challenging due to their diversity—numbering in the thousands depending on the expression system.
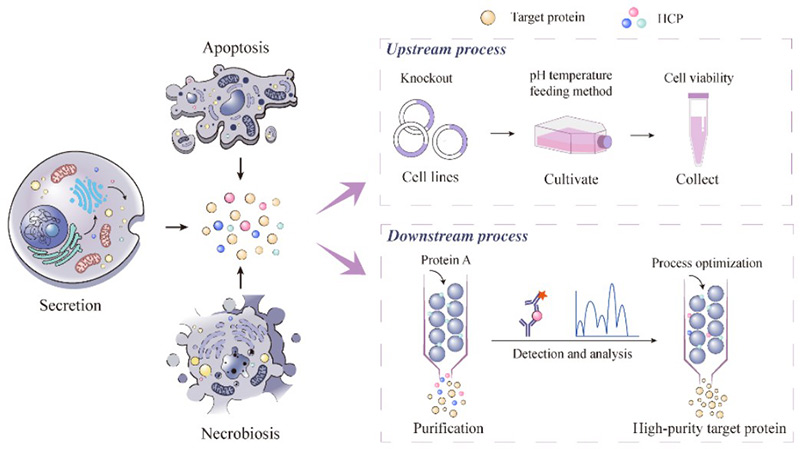
Figure 1. Source of host cell proteins (HCPs) and related upstream and downstream processes. (Zhao et al., 2024)
While some HCPs may be benign, others have the potential to induce immune responses, reduce drug efficacy, or interfere with stability and pharmacokinetics. Consequently, regulatory agencies require thorough characterization and minimization of HCPs in biologics before market approval.
However, detecting and mitigating HCPs is complex and requires a multi-disciplinary approach. No single assay is sufficient for comprehensive coverage. Instead, a combination of analytical tools is required to identify, quantify, and reduce HCP levels efficiently and accurately.
Creative BioMart addresses this challenge with a holistic, customizable service platform, offering unmatched expertise in both established and advanced analytical techniques. Our HCP Mitigation Services are designed to support biopharmaceutical developers at every stage—from discovery to late-stage clinical production.
What We Offer–Comprehensive HCP Mitigation Solutions for Biopharmaceuticals
Service Procedure

Service Details
We offer a full spectrum of state-of-the-art methods for HCP detection and quantification:
-
Protein Electrophoresis & Blotting
- 1D and 2D SDS-PAGE
- 1D/2D Western Blotting for HCPs and therapeutic proteins
-
Chromatography-Based Techniques
- SEC-HPLC: High Molecular Weight impurity analysis
- IEX-HPLC & RP-HPLC: Impurity separation, analysis, and collection
-
ELISA Services
- Evaluation of commercial HCP ELISAs
- Execution of commercial ELISA assays
- Custom development of product-specific or process-specific HCP ELISAs
- Validation of in-house or third-party ELISAs for regulatory compliance
-
Mass Spectrometry Approaches
- LC-MS/MS Proteomic Profiling: HCP identification and characterization
- LC-MRM Targeted Proteomics: Quantitative tracking of HCPs in in-process samples
All services are custom-tailored based on host cell line (CHO, E. coli, HEK293, etc.), production process, and product complexity.
Why Creative BioMart Leads in Host Cell Protein Removal
- Customized HCP Mitigation Strategy: Every project begins with a tailored analysis plan built around your expression system, process, and regulatory needs. No off-the-shelf solutions—just expert customization.
- Full Suite of Orthogonal Analytical Techniques: We deploy multiple, validated analytical platforms in parallel, ensuring the most comprehensive HCP coverage possible.
- Deep Expertise in Data Interpretation: Identifying a protein is one thing—knowing what it means for your drug's future is another. Our scientists provide actionable insights, not just raw data.
- Integrated Process Optimization: Beyond detection, we help mitigate HCPs by suggesting process improvements that reduce impurities at the source.
- Regulatory-Ready Documentation: We support your submissions with complete assay validations, SOPs, and regulatory consulting as needed for IND, BLA, or biosimilar applications.
- End-to-End Support: From discovery to late-stage development, we offer long-term HCP monitoring and method transfer support to your QC labs or CDMOs.
Effective HCP Mitigation: Success Stories in Industry
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Removal of residual DNA and host cell proteins for the purification of recombinant staphylokinase expressed in E. coli
Guang et al., 2024. doi:10.1002/sscp.202300214
Effective host cell protein (HCP) mitigation is critical in purifying biologics such as recombinant proteins and monoclonal antibodies. In the preparation of recombinant staphylokinase (r-SAK) expressed in E. coli, a multistep chromatography process—comprising anion exchange, cation exchange, and gel filtration—was developed to efficiently eliminate residual HCPs and host cell DNA (HCD). This method achieved residual HCP levels below 0.01% and HCD below 1 ng/mL, with final product purity exceeding 98%. Modified cellulose-based resins outperformed dextran and agarose matrices due to their structural strength, enabling stable, high-efficiency separation under demanding processing conditions. This highlights the importance of matrix integrity in HCP removal.
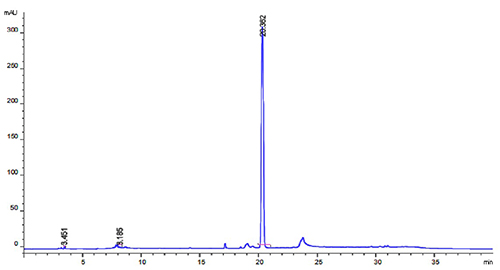
Figure 2. The HPLC of purified r-SAK protein. (Guang et al., 2024)
Case 2: Identification and characterization of CHO host-cell proteins in monoclonal antibody bioprocessing
Oh et al., 2024. doi:10.1002/bit.28568
Host cell proteins (HCPs) are critical process-related impurities that must be effectively mitigated during monoclonal antibody (mAb) manufacturing to ensure drug safety and stability. This study used mass spectrometry to monitor HCP profiles across downstream purification steps for seven mAbs derived from five different cell lines. Despite variability in overall HCP composition across processes, a core group of abundant and persistent HCPs was consistently identified in harvests. Results suggest that HCP abundance and specific interactions between mAbs and HCPs can contribute to purification resistance. These insights support targeted strategies for improving HCP clearance and refining purification processes in mAb production.
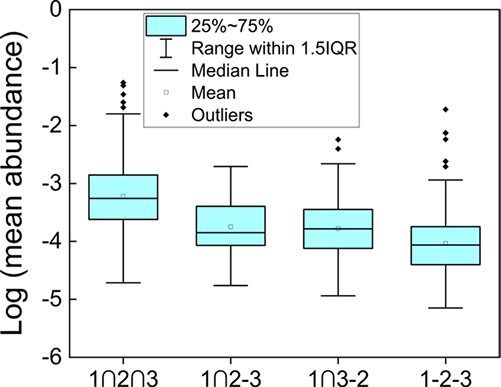
Figure 3. Statistical distribution of mean abundance of HCPs in mAb1 HCCF that are detected in all three mAbs, only two mAbs or one mAb among the HCPs quantified in mAb1. (Oh et al., 2024)
Client Feedback on Our Host Cell Protein Solutions
“We needed to assess and reduce residual HCPs in a monoclonal antibody produced in CHO cells for a late-stage clinical trial. Creative BioMart provided a complete solution—from ELISA development and validation to LC-MS/MS profiling—that not only pinpointed several immunogenic HCPs, but also helped us redesign our purification workflow. Their team was responsive, scientifically rigorous, and regulatory-savvy.”
— Director of Process Development | Biopharmaceutical Company
“Our team had limited in-house capability to evaluate host cell protein impurities in a recombinant enzyme expressed in E. coli. Creative BioMart stepped in with a comprehensive analytical package, including 2D Western blot and LC-MRM targeted quantification. Their interpretation of the results was incredibly helpful—we discovered specific process-related impurities that were impacting product stability. With their guidance, we adjusted our downstream process and saw a 70% reduction in HCP content.”
— Lead Scientist | Industrial Enzyme Manufacturer
“We were under pressure to meet regulatory expectations for HCP characterization in a fusion protein manufactured in HEK293 cells. Creative BioMart designed a tailored HCP mitigation strategy, including both a commercial ELISA and custom LC-MS/MS proteomic analysis. The report was thorough and submission-ready, with detailed insights that supported our CMC documentation. Their speed and technical depth saved us months of internal development time.”
— Regulatory Affairs Manager | Cell & Gene Therapy Startup
“As part of our biosimilar comparability assessment, we needed side-by-side HCP profiling between the reference product and our candidate. Creative BioMart delivered a full-spectrum analysis using RP-HPLC and mass spectrometry, helping us identify and match key HCP fingerprints. Their ability to run orthogonal methods and interpret subtle differences was exactly what we needed for confidence in our submission.”
— Senior R&D Scientist | Biosimilar Development Firm
Common Questions About Host Cell Protein Mitigation Services
-
Q: What types of host cell systems do you support for HCP analysis?
A: We support a wide range of expression systems including CHO, HEK293, E. coli, yeast, and insect cells. Our methods are adaptable to both mammalian and microbial hosts, and we offer system-specific assay development for accurate HCP detection and quantification. -
Q: Can you develop custom HCP ELISAs for our specific biotherapeutic product?
A: Yes. Creative BioMart specializes in developing and validating product-specific and process-specific HCP ELISAs, ensuring high sensitivity and specificity for your unique purification process and expression system. -
Q: How do you identify and quantify low-abundance or hard-to-detect HCPs?
A: We use orthogonal approaches, including LC-MS/MS, LC-MRM targeted proteomics, and 2D Western blotting, to detect even trace-level HCPs. These methods allow for deep profiling and accurate quantification, even for low-abundance or immunogenic proteins that may evade standard ELISAs. -
Q: Can you help interpret HCP data and recommend process improvements?
A: Absolutely. In addition to generating high-quality analytical data, our team of experts offers data interpretation and process optimization guidance—helping you reduce HCP load, refine purification workflows, and meet regulatory expectations with confidence. -
Q: Do your HCP mitigation services meet regulatory standards for IND/BLA submissions?
A: Yes. All our HCP mitigation services, including ELISA validation and mass spectrometry analysis, are designed to be regulatory-compliant and submission-ready, supporting your CMC package for IND, BLA, or biosimilar filings.
Resources
Related Services
- Biopharmaceutical Process and Product Related Impurities Analysis
- Protein Expression and Purification Services
- Protein Analytical Service
Related Products
References:
- Guang L, Jing L, Zhenxing Z, et al. Removal of residual DNA and host cell proteins for the purification of recombinant staphylokinase expressed in Escherichia coli. Separation Science Plus. 2024;7(9) :e202300214. doi:10.1002/sscp.202300214
- Oh YH, Mendola KM, C hoe LH, et al. Identification and characterization of CHO host ‐cell proteins in monoclonal antibody bioprocessing. Biotech & Bioengineering. 2024;121(1):291-305. doi:10.1002/bit.28568
- Zhao Y, Li H, Fan Z, Wang T. Effect of host cell protein on Chinese hamster ovary recombinant protein production and its removal strategies: a mini review. CPB. 2024;25(6):665-675. doi:10.2174/1389201024666230818112633
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

