Recombinant Human DBH protein(Ser26-Gly603), His-tagged
| Cat.No. : | DBH-210H |
| Product Overview : | Recombinant Human DBH (P09172) (Ser26-Gly603) was expressed in HEK293 with an N-terminal polyhistidine tag. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | Ser26-Gly603 |
| Form : | Lyophilized from sterile PBS, pH 7.4. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant human DBH comprises 598 amino acids and has a predicted molecular mass of 67.3 kDa. The apparent molecular mass of the protein is approximately 68 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C. Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | DBH dopamine beta-hydroxylase (dopamine beta-monooxygenase) [ Homo sapiens ] |
| Official Symbol | DBH |
| Synonyms | DBH; dopamine beta-hydroxylase (dopamine beta-monooxygenase); dopamine beta-hydroxylase; DBM; |
| Gene ID | 1621 |
| mRNA Refseq | NM_000787 |
| Protein Refseq | NP_000778 |
| MIM | 609312 |
| UniProt ID | P09172 |
| ◆ Cell & Tissue Lysates | ||
| DBH-869HCL | Recombinant Human DBH cell lysate | +Inquiry |
Case 1: Punchaichira TJ, et al. Neurogenetics. 2017
Dopamine β-hydroxylase (DBH) variants linked to neuropsychiatric and cardiovascular disorders exhibit impaired enzyme activity and secretion due to structural destabilization, as shown by HEK293 cell assays and molecular dynamics simulations. Experimental analysis of five prioritized nonsynonymous variants (e.g., R549C) revealed reduced homospecific activity and ER retention in L317P, underscoring their disrupted structure-function relationships. These findings advance understanding of DBH’s role in disease mechanisms and therapeutic targeting.
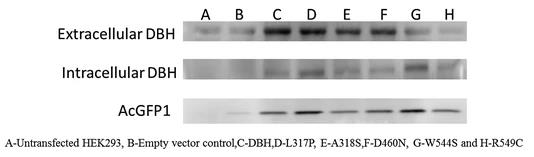
Fig1. Western blot showing differential secretion of L317P, W544S and R549C variants from transient HEK293 cell lines.
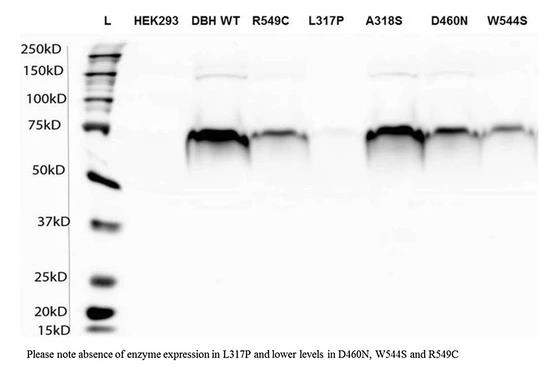
Fig2. Confirmation of expression of DBH in spent media from stable cell lines generated with DBH wild type and variants.
Case 2: Liu J, et al. Neurobiol Stress. 2024
Dopamine β-hydroxylase knockout (Dbh -/-) mice exhibit altered predator odor responses, replacing defensive burying with excessive grooming due to disrupted norepinephrine (NE) signaling and elevated dopamine (DA). NE-blocking drugs suppress burying in controls, while DA antagonists normalize grooming in Dbh -/- mice. Neural activity mapping revealed reduced c-fos in stress-regulating regions (e.g., anterior cingulate cortex) and increased activity in arousal-related areas (locus coeruleus), highlighting NE-DA interplay in innate defensive behaviors and anxiety-related neural circuits.
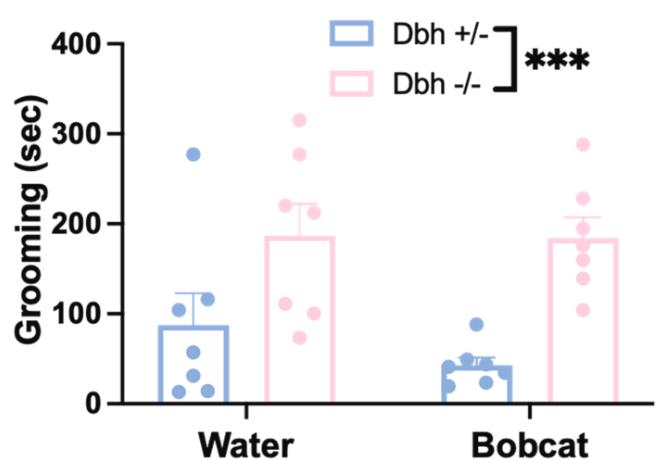
Fig1. Effects of DBH knockout on behavioral responses to predator odor.
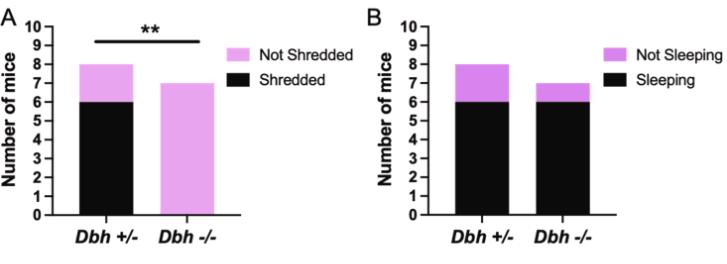
Fig2. Dbh+/− and Dbh −/− mice were exposed to lavender oil.
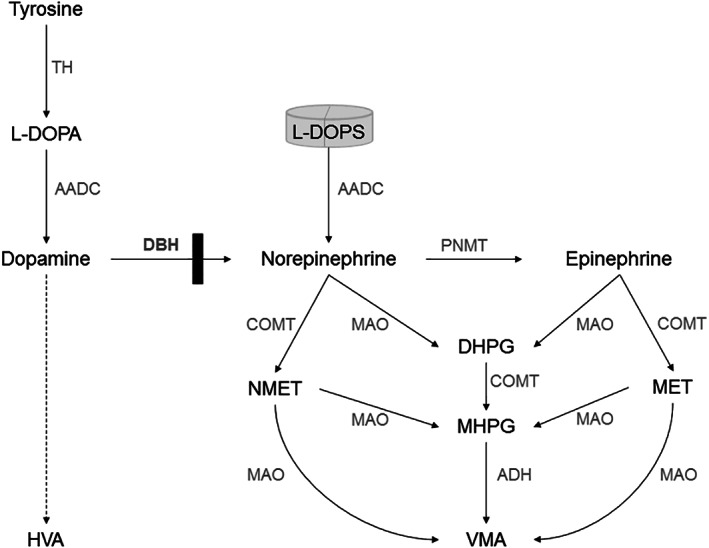
Fig1. Simplified scheme of catecholamine synthesis and breakdown in DBH‐deficiency. (Tessa Wassenberg, 2021)
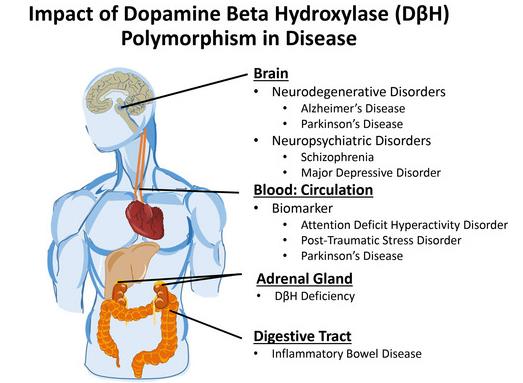
Fig2. The role of genetic variants in DβH and its role in health and disease. (Eugene Gonzalez-Lopez, 2020)
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All DBH Products
Required fields are marked with *
My Review for All DBH Products
Required fields are marked with *



