Recombinant Mouse Cdk1, His-GST tagged
| Cat.No. : | CDK1-402M |
| Product Overview : | Recombinant Mouse Cdk1 (P11440) (Met1-Met297), fused with the N-terminal polyhistidine-tagged GST tag, was produced in Baculovirus-Insect cells. |
| Availability | February 09, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Mouse |
| Source : | Insect Cells |
| Tag : | Fc |
| Protein Length : | 1-297 a.a. |
| Predicted N Terminal : | Met |
| Form : | Lyophilized from sterile 20mM Tris, 500mM Nacl, 10% glycerol, pH 8.0.Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. |
| Molecular Mass : | The recombinant mouse CDK1/GST chimera consists of 534 amino acids and has a calculated molecular mass of 61.9 kDa. The recombinant protein migrates as an approximately 57 kDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 eu per μg of the protein as determined by the LAL method. |
| Purity : | >85 % as determined by SDS-PAGE |
| Stability : | Samples are stable for up to twelve months from date of receipt at -70oC. |
| Storage : | Store it under sterile conditions at -20oC~-70oC. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution. Centrifuge the vial at 4℃ before opening to recover the entire contents. |
| Publications : |
| Gene Name | Cdk1 cyclin-dependent kinase 1 [ Mus musculus ] |
| Official Symbol | Cdk1 |
| Gene ID | 12534 |
| mRNA Refseq | NM_007659 |
| Protein Refseq | NP_031685 |
| ◆ Recombinant Proteins | ||
| CDK1/CyclinA2-271H | Recombinant Human CDK1/Cyclin A2, GST-tagged, Active | +Inquiry |
| CDK1-9706HF | Active Recombinant Full Length Human CDK1 Protein, GST-tagged | +Inquiry |
| CDK1-2211M | Recombinant Mouse CDK1 protein, His&GST-tagged | +Inquiry |
| CDK1-957R | Recombinant Rat CDK1 Protein, His (Fc)-Avi-tagged | +Inquiry |
| CDK1-3143H | Recombinant Human CDK1 Protein, MYC/DDK-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CDK1-670HCL | Recombinant Human CDK1 cell lysate | +Inquiry |
| CDK1-001MCL | Recombinant Mouse CDK1 cell lysate | +Inquiry |
Cyclin B1 is essential for mitosis in mouse embryos, and its nuclear export sets the time for mitosis
Journal: The Journal of Cell Biology PubMed ID: 29074707 Data: 2018/1/2
Authors: Bernhard Strauss, Andrew Harrison, Jonathon Pines
Article Snippet:Recombinant mouse His-GST CDK1 and recombinant mouse 10xHis CCNB1Myc (Creative BioMart) were used as protein standards and loaded at 1, 5, 10 ng per lane.. Wild-type mouse embryo samples containing 130 uninjected embryos and 130 embryos injected with CyclinB1–Venus mRNA at the zygote stage were harvested at the two-cell stage, and embryo extracts were prepared using lysis buffer (150 mM KCl, 20 mM Hepes, pH 7.6, 2 mM EGTA, 1.5 mM MgCl 2 , 50 mM NaF, 0.1% NP-40, 10% glycerol, 1 mM Na3VO4, 20 mM β-glycerophosphate, 1 mM dithiothreitol, 10 mM benzamidine HCl, and 25 U/ml Benzonase nuclease [Merck]) supplemented with Protease inhibitor cocktail (P2714; Sigma-Aldrich).Wild-type mouse embryo samples containing 130 uninjected embryos and 130 embryos injected with CyclinB1–Venus mRNA at the zygote stage were harvested at the two-cell stage, and embryo extracts were prepared using lysis buffer (150 mM KCl, 20 mM Hepes, pH 7.6, 2 mM EGTA, 1.5 mM MgCl 2 , 50 mM NaF, 0.1% NP-40, 10% glycerol,..
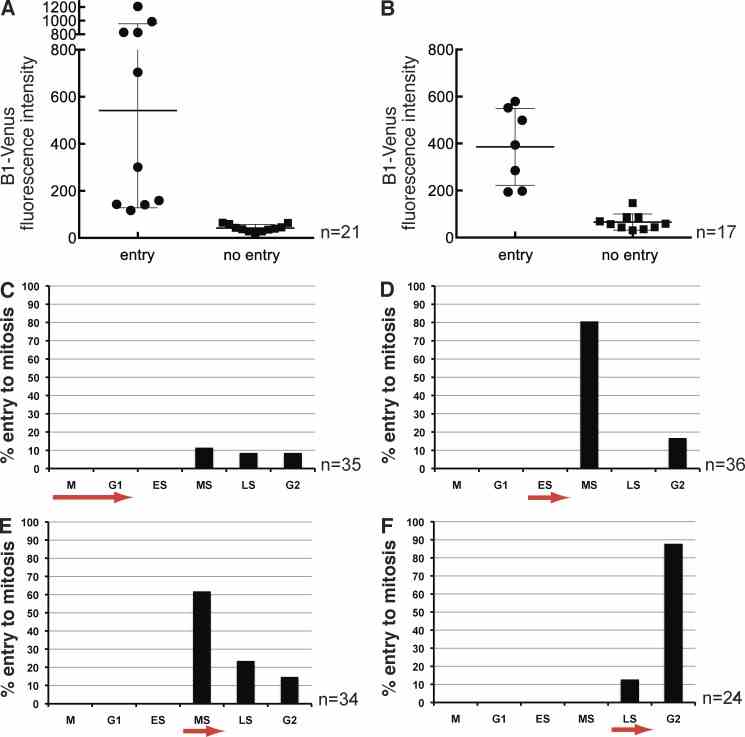
Low amounts of Cyclin B1 are sufficient to trigger mitosis and the threshold is reached by mid S phase. (A) To establish the threshold of Cyclin B1–Venus that can drive null cells into mitosis, fluorescence intensity was used as a read-out for the amount of protein and measured in individual blastomeres of ?/? embryos; y axis arbitrary intensity units. The threshold concentration to trigger mitosis lies between 100 and 200 units above background intensity, corresponding to ~1/10 of the sampled intensity range ( n = 21 blastomeres from five experiments). (B) Wild-type Cyclin B1–Venus was injected into one blastomere of ?/? embryos at the two-cell stage to rescue the arrest phenotype. Signal intensity was measured at the 8- to 16-cell transition after addition of Wee1 inhibitor. Only injected cells with a Cyclin B1 level above a threshold of ~150 arbitrary units can be forced into mitosis by Wee1 inhibition; n = 17, 1 experiment. Error bars are the SD of the mean. (C–F) To test whether there is a time dependency of the inhibitory phosphorylation of
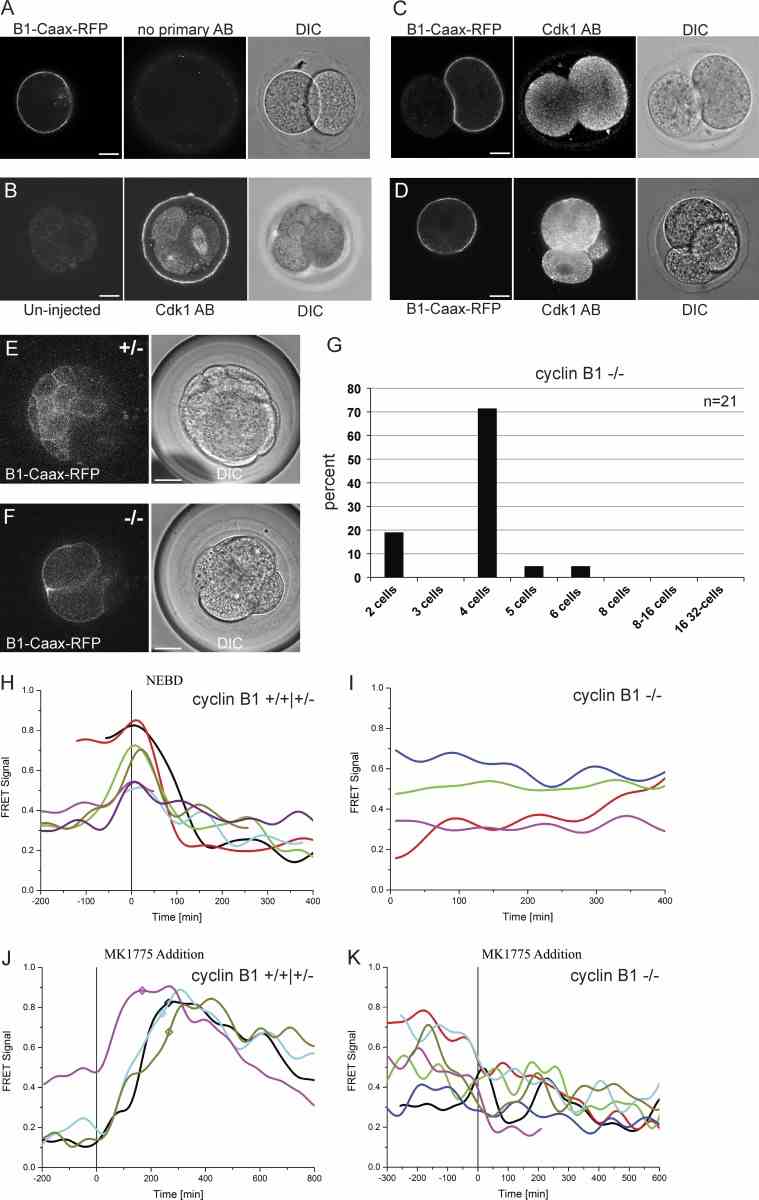
Tethering Cyclin
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All Cdk1 Products
Required fields are marked with *
My Review for All Cdk1 Products
Required fields are marked with *



