Colon Cancer Biomarkers
Related Symbol Search List
- ATAD2
- CEACAM5
- TMEM25
- WISP3
- AOC3
- DKK1
- GALNT12
- GPA33
- GRN
- MFAP3L
- PDGFRL
- PSG2
- PSG3
- REG4
- SAA1
- TPSAB1
- UVRAG
- 14-3-3 beta
- YWHAZ
Immunology Background
What is gastrointestinal cancer
Gastrointestinal (GI) cancer is a term for the group of cancers that affect the digestive system. Gastrointestinal (GI) cancer is one of foremost health concerns in the world between all cancers and the loads are growing in several nations. Esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC) are the major malignancies of gastrointestinal cancer. EC is the sixth leading cause of cancer-related death worldwide, GC is the third and CRC is the fifth. Although the overall survival of patients with gastrointestinal cancer has improved due to growing early detection and more widespread implementation of radical surgery, plenty of patients with gastrointestinal cancer continue to be diagnosed at advanced stages and therefore lose the optimal opportunity for radical cure. Therefore, searching for ideal diagnostic and prognostic biomarker is significant to improve the curative effect of gastrointestinal cancer.
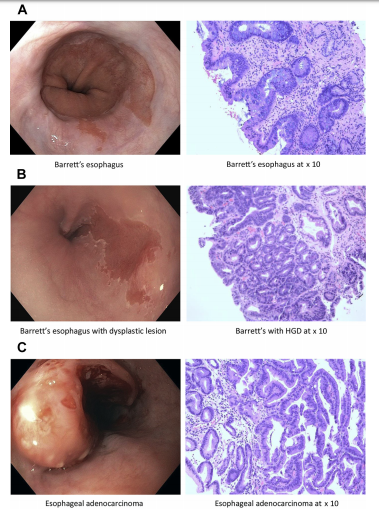 Figure 1.. Endoscopic and microscopic appearance of Barrett’s esophagus. (A) Barrett’s esophagus. (B) Barrett’s esophagus with dysplastic lesion. (C) Esophageal adenocarcinoma. (A–C, H&E stain, original magnification 10).
Figure 1.. Endoscopic and microscopic appearance of Barrett’s esophagus. (A) Barrett’s esophagus. (B) Barrett’s esophagus with dysplastic lesion. (C) Esophageal adenocarcinoma. (A–C, H&E stain, original magnification 10).
Types of gastrointestinal cancer
Gastrointestinal cancer includes cancers of the esophagus, gallbladder, liver, pancreas, stomach, small intestine, bowel (large intestine or colon and rectum), and anus. Generally,gastrointestinal cancer is classified into three main types: esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC). Esophageal cancer has two common histologic types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC).
Risk factors for gastrointestinal cancer
Diet is an important variable in cancer etiology and prevention. Dietary risk factors for GI cancers are similar with the major cause being consumption of smoked, pickled, spicy, and salted food; fermented meat, high-alkaline foods, intake of foods at high temperature, prolonged storage, and processing of red meat. Alcohol was also found to be a major risk factor among GI cancers. Deficit of micronutrients such as selenium; zinc; and vitamins A, B2, C, and E in the diet could aggravate GI cancer risk. Major nondietary risk factors for stomach cancer are H. pylori infection and hepatitis B and C for liver cancer. Tobacco in any form is a well-known risk factor which acts independently or enhances the effect of other risk factors in GI cancers. Body mass index has shown difference in the cancer cell type, wherein low BMI is a risk factor for esophageal squamous cell carcinomas and high BMI in esophageal adenocarcinomas. In women with higher BMI, intake of diets high in MUFA and PUFA have also shown a close link with colorectal cancer, and hence, BMI above the normal range is a non-dietary risk factor for cancers of the esophagus and colorectum.
Gastrointestinal cancer proteins
Biomarkers are features of objective measurement and assessment that can be used as indicators of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic interventions. Various biomarkers have been used in clinical practice for early detection and treatment monitoring of cancer. Cancer antigen 125 (CA-125; in ovarian cancer), CA 15-3 (in breast cancer), carcinoembryonic antigen (CEA; in colon cancer) and prostate-specific antigen (in prostate cancer) are some examples of cancer biomarkers. For early diagnosis, several biomarkers, such as CA72-4, alpha-fetoprotein, CEA, CA 19-9, and CA125, were used in gastric cancer. Serum levels of CEA and CA 19-9 have been used as biomarkers for the diagnosis of esophageal adenocarcinoma(EAC). CEA, SCC-Ag, and CYFRA21-1 have been used as tumor biomarkers for the diagnosis of esophageal squamous cell carcinoma(ESCC). p53 antibody induced by mutant p53 protein has been used as biomarker for both EAC and ESCC.
Recently, researches showed that overexpression of Yes-associated protein 1 (YAP or YAP1), an oncoprotein encoded by the YAP1 gene located on the human chromosome 11q22, correlates with shorter OS in gastrointestinal cancer, especially in Asian patients. Furthermore, YAP1 has been considered to be a promising target for therapy of gastrointestinal cancer.
Table 1. Protein biomarkers for the detection of gastrointestinal cancer
| Types of gastrointestinal cancer | biomarkers |
| EC | C-reactive protein (CRP), Anoctamin 1 (ANO1), Angiopoietin-Like Protein 2 |
| GC | Glucose-regulated protein 78(GRP78) , Endothelial lipase protein (EL protein) , CA72-4 , afamin , apoE , clusterin , haptoglobin , CEA , CA19-9 |
| CRC | Lectin adhesion proteins (P-, L- and E-selectins), Interleukin-6 protein , leucine-rich PPR-motif-containing protein (LRPPRC) |
References:
1. Zhang L, Song X, Li X, et al. Yes-Associated Protein 1 as a Novel Prognostic Biomarker for Gastrointestinal Cancer: A Meta-Analysis[J]. BioMed research international, 2018.
2. Dong X , Wang G , Zhang G , et al. The endothelial lipase protein is promising urinary biomarker for diagnosis of gastric cancer[J]. Diagnostic Pathology, 2013, 8(1):45.
3. Harada K, Mizrak Kaya D, Shimodaira Y, et al. Proteomics approach to identify biomarkers for upper gastrointestinal cancer[J]. Expert review of proteomics, 2016, 13(11): 1041-1053.

