Pichia Glyco Protein Production
Creative BioMart offers advanced Pichia Glycoprotein Production services , enabling the efficient expression of therapeutic glycoproteins with customized human-like glycosylation. Leveraging engineered strains of Pichia pastoris , a robust and scalable eukaryotic host, we produce glycoproteins with high yield, consistent quality, and tailored glycosylation profiles. Our platform combines the ease and speed of microbial systems with the complex protein processing capabilities of higher eukaryotes—making it ideal for biopharmaceutical development, structural biology, and functional studies. Whether you need uniform glycoforms or optimized therapeutic activity, our service ensures cost-effective, high-quality glycoprotein production that meets your specific research or manufacturing needs.
Background: Why Choose Pichia pastoris for Glycoprotein Expression
What Is Pichia pastoris and Why Use It
Pichia pastoris is a methylotrophic yeast and a widely used system for eukaryotic protein expression. It offers the advantages of post-translational modifications, including protein folding, disulfide bond formation, and glycosylation, similar to higher eukaryotic systems. However, unlike complex systems such a s mammalian cell culture or baculovirus expression , Pichia is significantly faster, easier, and more cost-effective. It also supports high cell densities, enabling exceptionally high protein yields in shorter production cycles.
The Importance of Glycosylation in Therapeutic Proteins
Many therapeutic proteins—such as monoclonal antibodies , enzymes, and cytokines —are glycoproteins, requiring the attachment of sugar moieties for proper function. Glycosylation influences protein folding, stability, solubility, pharmacokinetics, and biological activity. However, conventional mammalian expression systems often produce heterogeneous glycoforms, leading to batch-to-batch variability, reduced efficacy, or undesired immune responses.
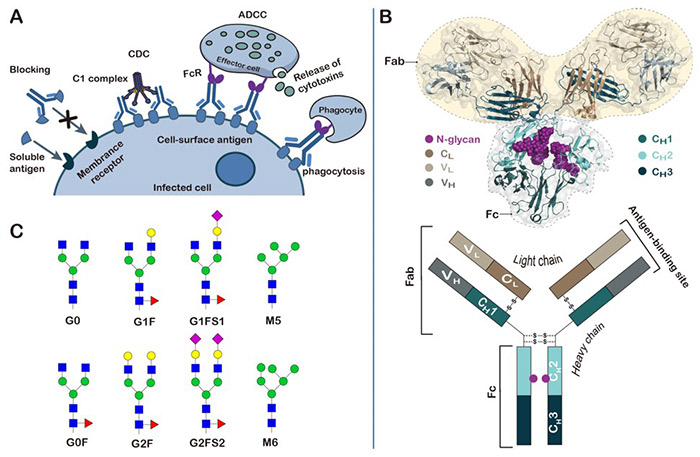
Figure 1. Structure, function and glycosylation of antibodies. (Chen et al ., 2022)
Our Pichia Glycoengineering Platform
Creative BioMart employs a glycoengineered Pichia platform that has been modified to enable human-like glycosylation. This allows for the expression of proteins with uniform, customizable glycoforms, improving homogeneity, therapeutic function, and regulatory consistency. Our system minimizes aberrant or immunogenic glycosylation patterns typically found in yeast, ensuring your biopharmaceutical candidates are optimized for downstream development.
Our Service Offering for Pichia Glycoprotein Production
Service Procedure
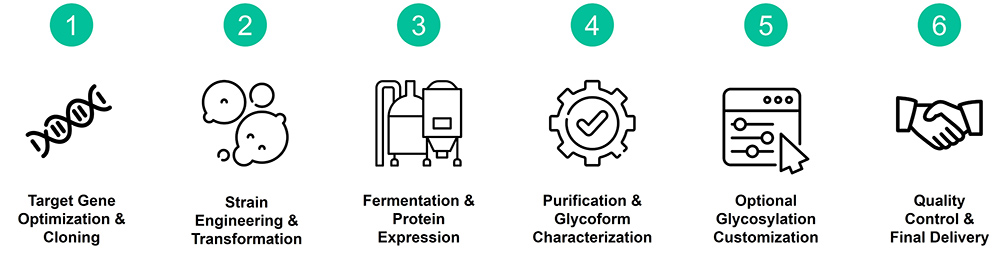
Service Details
|
Item |
Details |
|---|---|
|
Host System |
Glycoengineered Pichia pastoris |
|
Glycoform Customization |
Human-like, uniform, or client-defined |
|
Expression Scale |
Milligram to multigram production |
|
Delivery Format |
Purified protein in buffer of choice |
|
QC Options |
Glycan analysis, purity, activity, endotoxin, and more |
|
Turnaround Time |
4–8 weeks depending on complexity and scale |
|
Applications |
Biologics development, vaccine candidates, biosimilars, structural studies |
Advantages of Our Pichia Glycoprotein Production
- Uniform Glycosylation : Achieve consistent glycan profiles for higher batch reproducibility.
- High Expression Yields : Produce milligram to gram quantities efficiently and cost-effectively.
- Customized Glycosylation : Tailor sugar structures to match functional or therapeutic needs.
- Reduced Aberrant Glycoforms : Engineered strains minimize non-human or immunogenic sugars.
- Increased Homogeneity : Essential for downstream development and regulatory approval.
- Faster Turnaround, Lower Cost : Compared to mammalian or insect cell systems.
Case Studies: Real-World Results with Pichia Glycoprotein Production
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Optimization of humanized IgGs in glycoengineered Pichia pastoris
Li et al. , 2006. doi:10.1038/nbt1178
Monoclonal antibodies (mAbs) are the fastest-growing class of therapeutic proteins, and their native human forms are glycosylated. While clinically approved mAbs are typically produced in mammalian cells, these systems generate glycoforms that differ slightly from natural human structures. Glycosylation critically impacts mAb interactions with immune effector cells, influencing therapeutic efficacy. This study demonstrates that glycoengineered Pichia pastoris can produce human antibodies with defined human-like N-glycan structures. By tailoring specific glycoforms, antibody-mediated immune functions can be optimized. This establishes glycoengineered P. pastoris as a versatile and scalable platform for producing recombinant mAbs with customized, human-compatible glycosylation.
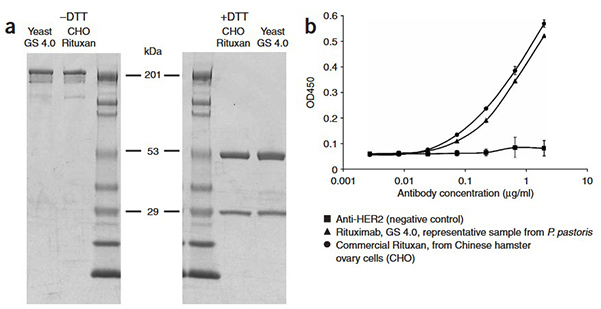
Figure 2. Characterization of yeast-derived antibody. SDS-PAGE of commercial rituximab, that is, Rituxan and rituximab derived from glycoengineered yeast. (a) Nonreducing gel (left panel) and reducing gel (right panel). (b) Binding of rituximab variants to CD20 antigen on WIL2-S cells. (Li et al ., 2006)
Case 2: The SARS-CoV-2 receptor-binding domain expressed in Pichia pastoris as a candidate vaccine antigen
Limonta-Fernández et al. , 2022. doi:10.1016/j.nbt.2022.08.002
This study used the Pichia pastoris yeast system to produce a chimeric S1 protein fused with a human Fc domain (S1Fc), chosen for its low cost and scalability compared to mammalian or insect cells. The S1Fc construct, consisting of 918 amino acids, was expressed in both standard and glycoengineered (GlycoSwitch) Pichia strains. Both showed successful expression, with the GlycoSwitch strain yielding more native-like glycosylation and enhanced antigenicity. These results support Pichia pastoris as a promising, efficient platform for producing recombinant proteins for vaccine development.
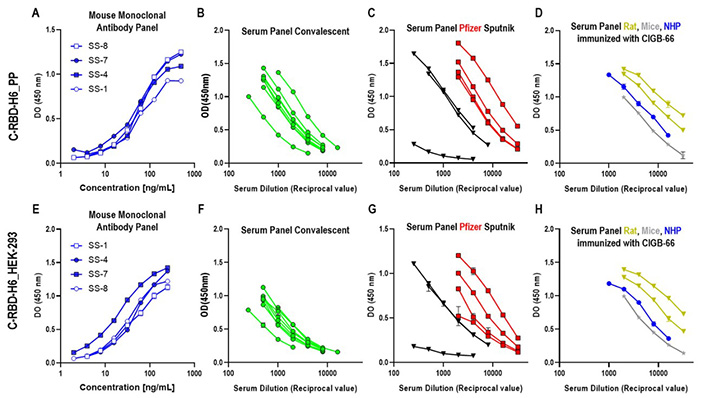
Figure 3. C-RBD-H6 PP (upper panel) or RBD-H6 HEK (lower panel) proteins were used for coating ELISA plates and for animal immunization. Sera and monoclonal antibodies were used in serial two-fold dilutions. (A,E). SS-1, SS4, SS-7 and SS-8 monoclonal antibodies; (B,F). COVID-19 sera from convalescents; (C,G) Sera from subjects immunized with Pfizer-BioNTech (red squares) and Sputnik vaccines (black triangles); (D,H). Mice (grey) and NHP (blue) sera. (Limonta-Fernández et al ., 2022)
What Our Clients Say About Our Pichia Glycoprotein Production
“We partnered with Creative BioMart to produce a glycoengineered version of an IgG1 antibody for our oncology pipeline. Their Pichia platform delivered outstanding expression yields and uniform glycosylation, helping us avoid the batch-to-batch variability we were seeing in CHO cell production. The ability to fine-tune glycoforms was key to enhancing Fc effector function. The entire project—from gene cloning to final QC—was completed ahead of schedule.”
— R&D Director | Mid-Sized Biopharmaceutical Company
“We needed a high-throughput way to produce glycoprotein antigens for our COVID-19 subunit vaccine study. Creative BioMart’s glycoengineered Pichia pastoris system allowed us to generate several variants of the S1-Fc fusion protein with controlled glycosylation. Their engineered strain in particular gave us the closest match to mammalian-like glycans, which significantly boosted antigenicity in ELISA assays. Their technical team provided excellent support in vector design and yield optimization.”
— Senior Scientist | Academic Infectious Disease Lab
“We needed a glycosylated lysosomal enzyme for early-stage testing in an enzyme replacement therapy program. The Creative BioMart team engineered a custom Pichia strain to produce a homogeneously glycosylated version of the enzyme with improved plasma stability. Not only did we get consistent batches, but their team also provided in-depth glycan analysis, which helped us in our preclinical documentation.”
— Project Manager | Rare Disease Biotech Firm
“Our client required gram-scale production of a recombinant cytokine with human-like glycosylation for functional studies. Creative BioMart’s Pichia Glyco platform was the perfect fit. We were impressed by their fast turnaround, high expression yield, and consistent glycoform profile across multiple production lots. Their fermentation and purification processes were clearly well-optimized. This service saved us time and budget compared to CHO-based approaches.”
— Laboratory Head | Contract Research Organization (CRO)
Frequently Asked Questions– Pichia Glycoprotein Production
-
Q: What is glycoengineered Pichia pastoris , and why is it beneficial for glycoprotein production?
A: Glycoengineered Pichia pastoris strains, have been modified to produce human-like N-glycans, overcoming one of the main limitations of yeast systems. This enables the production of glycoproteins with enhanced biological activity, improved stability, and increased therapeutic relevance, especially for antibodies, enzymes, and vaccine antigens. -
Q: How does your Pichia -based service compare to mammalian cell expression?
A: Our Pichia pastoris platform offers faster timelines, higher expression levels, and lower production costs than traditional mammalian systems like CHO. More importantly, our glycoengineering technology minimizes undesired glycoforms and delivers consistent, customizable glycosylation patterns—ideal for biopharmaceutical development. -
Q: Can you produce glycoproteins with uniform glycosylation?
A: Yes. One of the key advantages of our Pichia Glycoprotein Production service is uniform glycosylation. Unlike mammalian cells, which often generate heterogeneous glycoforms, our engineered Pichia strains provide more homogeneous glycan profiles, improving product consistency and functional performance. -
Q: What kinds of proteins are best suited for production in your Pichia system?
A: Our service is ideal for a wide range of therapeutic and research-grade glycoproteins, including monoclonal antibodies, Fc-fusion proteins, cytokines, enzymes, and vaccine antigens. The platform is particularly valuable when glycosylation impacts protein activity, stability, or immunogenicity. -
Q: How do you ensure the quality and consistency of the glycoproteins produced?
A: We use rigorous QC testing, including SDS-PAGE, Western blot, mass spectrometry, and glycan profiling to verify expression, purity, and glycosylation. All proteins are produced under controlled fermentation conditions with batch-to-batch reproducibility in mind. -
Q: Can you customize glycosylation patterns for my specific application?
A: Absolutely. We offer glycosylation customization services to align with your desired functional or pharmacokinetic profile. Whether you need sialylated, high-mannose, or hybrid N-glycans, we can tailor the expression strain and processing to meet your needs.
Resources
Related Services
- Yeast Display Platform
- Yeast Expression Systems
- Recombinant Antibody Production Services
- Protein Engineering Services
- Protein Expression and Purification Services
- Endotoxin Removal Service
- Protein Glycosylation Labeling Service
- Mammalian Expression Systems
- Baculovirus-Insect Cell Expression
Related Products
References:
- Chen B, Liu W, Li Y, Ma B, Shang S, Tan Z. Impact of N-linked glycosylation on therapeutic proteins. Molecules . 2022;27(24):8859. doi:10.3390/molecules27248859
- Li H, Sethuraman N, Stadheim TA, et al . Optimization of humanized IgGs in glycoengineered Pichia pastoris . Nat Biotechnol . 2006;24(2):210-215. doi:10.1038/nbt1178
- Li X, Shen J, Chen X, et al . Humanization of yeasts for glycan-type end-products. Front Microbiol . 2022;13:930658. doi:10.3389/fmicb.2022.930658
- Limonta-Fernández M, Chinea-Santiago G, Martín-Dunn AM, et al . An engineered SARS-CoV-2 receptor-binding domain produced in Pichia pastoris as a candidate vaccine antigen. New Biotechnology . 2022;72:11-21. doi:10.1016/j.nbt.2022.08.002
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

