Recombinant Mouse Ace2 protein, His-tagged
| Cat.No. : | Ace2-761M |
| Product Overview : | Recombinant Mouse Ace2 protein(Gln 18 - Thr 740), fused with His tag, was expressed in HEK293. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Mouse |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | Gln 18 - Thr 740 |
| Form : | Supplied as 0.2 μm filtered solution in 50 mM Tris, 150 mM NaCl, Arginine, pH7.5 with glycerol as protectant. |
| Molecular Mass : | This protein carries a polyhistidine tag at the N-terminusThe protein has a calculated MW of 85.4 kDa. The protein migrates as 90-110 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation. |
| Endotoxin : | Less than 1.0 EU per μg by the LAL method. |
| Purity : | >95% as determined by SDS-PAGE. >90% as determined by SEC-MALS. |
| Storage : | For long term storage, the product should be stored at lyophilized state at -20°C or lower.Please avoid repeated freeze-thaw cycles. This product is stable after storage at: -20°C to -70°C for 12 months in lyophilized state; -70°C for 3 months under sterile conditions after reconstitution. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | Ace2 angiotensin I converting enzyme (peptidyl-dipeptidase A) 2 [ Mus musculus ] |
| Official Symbol | Ace2 |
| Synonyms | ACE2; angiotensin I converting enzyme (peptidyl-dipeptidase A) 2; angiotensin-converting enzyme 2; ACE-related carboxypeptidase; angiotensin I converting enzyme 2; 2010305L05Rik; |
| Gene ID | 70008 |
| mRNA Refseq | NM_001130513 |
| Protein Refseq | NP_001123985 |
| ◆ Recombinant Proteins | ||
| ACE2-031H | Recombinant Human ACE2 Protein, Fc-tagged | +Inquiry |
| ACE2-1368H | Recombinant Human ACE2 protein(Met1-Ser740), hFc-tagged, Biotinylated | +Inquiry |
| ACTN4-2451H | Recombinant Human ACTN4 protein, His-tagged | +Inquiry |
| ACE2-38H | Recombinant Human ACE2 Protein, hFc-tagged | +Inquiry |
| ACE2-26H | Recombinant Human ACE2 Protein (ECD, processed, AA 20-708), Tag-free | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| ACE2-3100HCL | Recombinant Human ACE2 cell lysate | +Inquiry |
| ACE2-1851RCL | Recombinant Rat ACE2 cell lysate | +Inquiry |
| ACE2-2085MCL | Recombinant Mouse ACE2 cell lysate | +Inquiry |
| ACE2-887CCL | Recombinant Cynomolgus ACE2 cell lysate | +Inquiry |
CXCL12 and CXCL13 Cytokine Serum Levels Are Associated with the Magnitude and the Quality of SARS-CoV-2 Humoral Responses
Journal: Viruses PubMed ID: 36560669 Data: 2022/11/28
Authors: Alessandra Noto, Victor Joo, Caijun Sun
Article Snippet:Control wells were included on each 96-well plate that included beads alone, matching the serum dilutions of a control pool of pre-COVID-19 pandemic healthy human sera (BioWest human serum AB males; VWR) and a positive control of commercial anti-Spike blocking antibody (SAD-S35, from ACRO Biosystem, Newark, DE, USA ) or recombinant-produced REGN10933 neutralizing antibody, discovered and marketed by Regeneron and tested in a concentration response.pool of pre-COVID-19 pandemic healthy human sera (BioWest human serum AB males; VWR) and a positive control of commercial anti-Spike blocking antibody (SAD-S35, from ACRO Biosystem, Newark, DE, USA ) or recombinant-produced REGN10933 neutralizing antibody, discovered and marketed by Regeneron and tested in a concentration response. ... The plates were agitated on a plate shaker for 60 min, then the ACE-2 mouse Fc fusion protein (either Creative BioMart or produced by the EPFL Protein Production and Structure Core Facility) was then added to each well, at a final concentration of 1 μg/mL, and agitated for a further 60 min.. The beads were then washed with the magnetic plate washer, then anti-mouse IgG-PE secondary antibody (OneLambda, Thermo Fisher) was added at a 1/100 dilution, with 50 μL per well.The beads were then washed with the magnetic plate washer, then anti-mouse IgG-PE secondary antibody (OneLambda, Thermo Fisher) was added at a 1/100 dilution, with 50 μL per well.
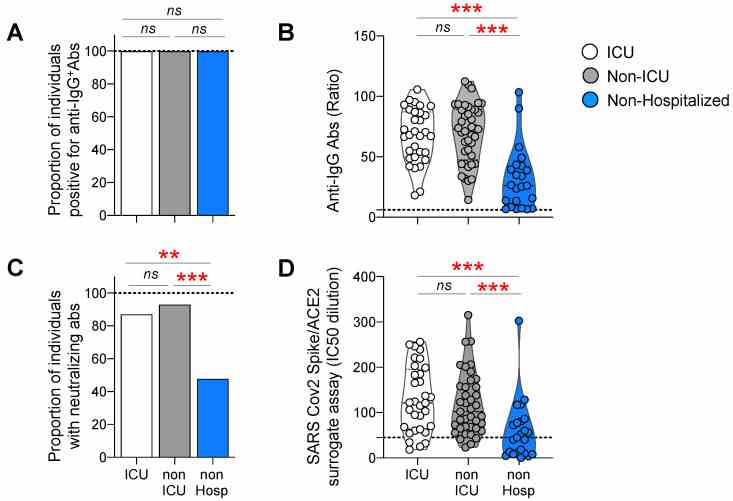
Antibody responses against the Spike protein in ICU, non-ICU, and non-hospitalized individuals. Luminex beads, coupled with Spike proteins, were used to monitor the IgG binding antibody response; neutralization was measured by a surrogate
A multiplexed high-throughput neutralization assay reveals a lack of activity against multiple variants after SARS-CoV-2 infection
Journal: medRxiv Data: 2021/4/13
Authors: Fenwick Craig, Turelli Priscilla, Trono Didier
Article Snippet:PrePrint: Control wells were included on each 96-well plate that included beads alone, matching serum dilutions of a control pool of pre-COVID-19 pandemic healthy human sera (BioWest human serum AB males; VWR) and a positive control commercial anti-Spike blocking antibody (SAD-S35 from ACRO Biosciences) or recombinant produced REGN10933 neutralizing antibody discovered and marketed by Regeneron tested in a concentration response.serum dilutions of a control pool of pre-COVID-19 pandemic healthy human sera (BioWest human serum AB males; VWR) and a positive control commercial anti-Spike blocking antibody (SAD-S35 from ACRO Biosciences) or recombinant produced REGN10933 neutralizing antibody discovered and marketed by Regeneron tested in a concentration response. ... Plates were agitated on a plate shaker for 60 minutes then the ACE2 mouse Fc fusion protein (Creative BioMart or produced by EPFL Protein Production and Structure Core Facility) was then added to each well at a final concentration of 1 μg/ml and agitated for a further 60 minutes.. Beads were then washed on the magnetic plate washer and anti-mouse IgG-PE secondary antibody (OneLambda ThermoFisher) was added at a 1/100 dilution with 50μl per well.Beads were then washed on the magnetic plate washer and anti-mouse IgG-PE secondary antibody (OneLambda ThermoFisher) was added at a 1/100 dilution with 50μl per well.
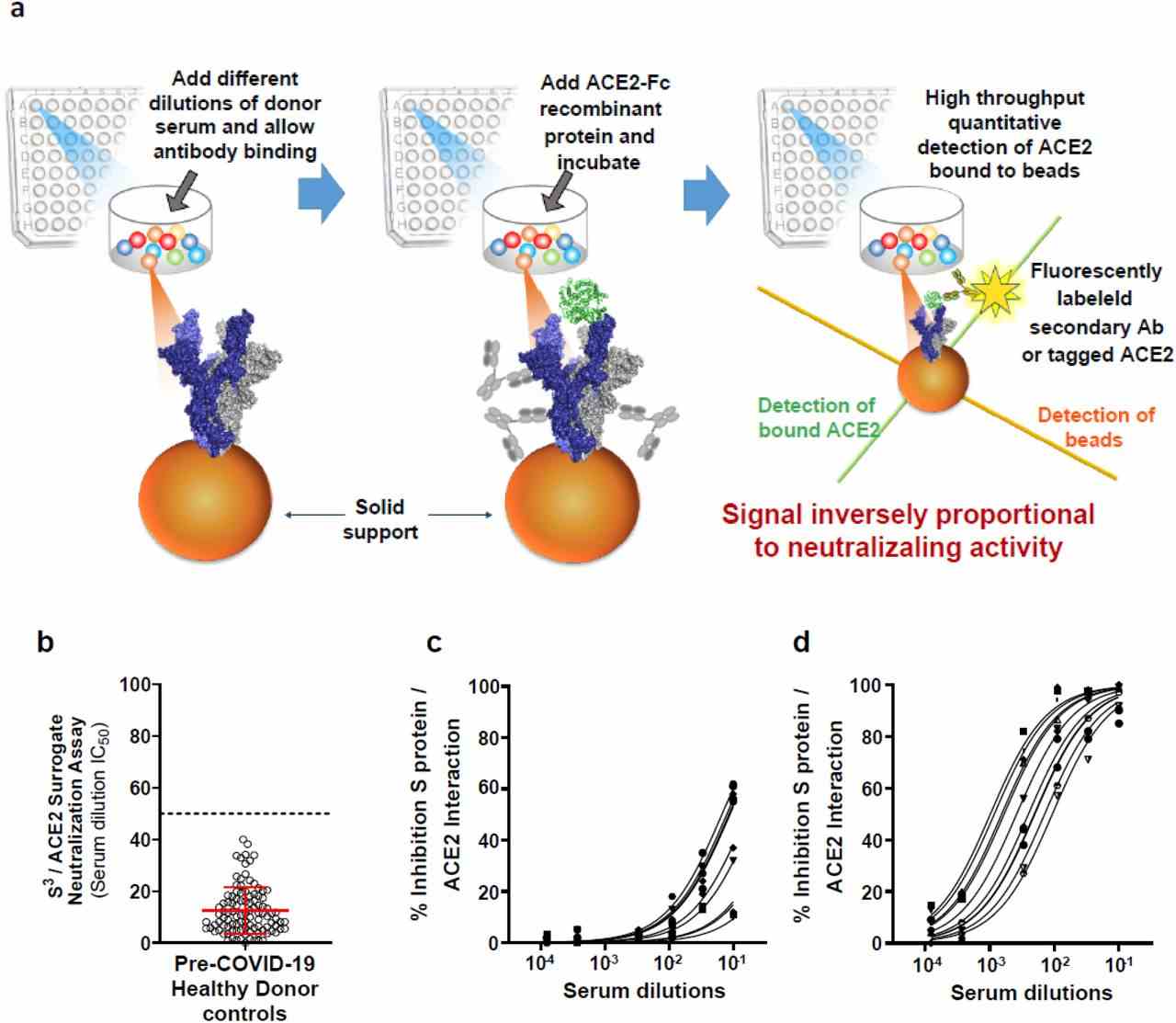
( a ) Schematic outline of the S 3
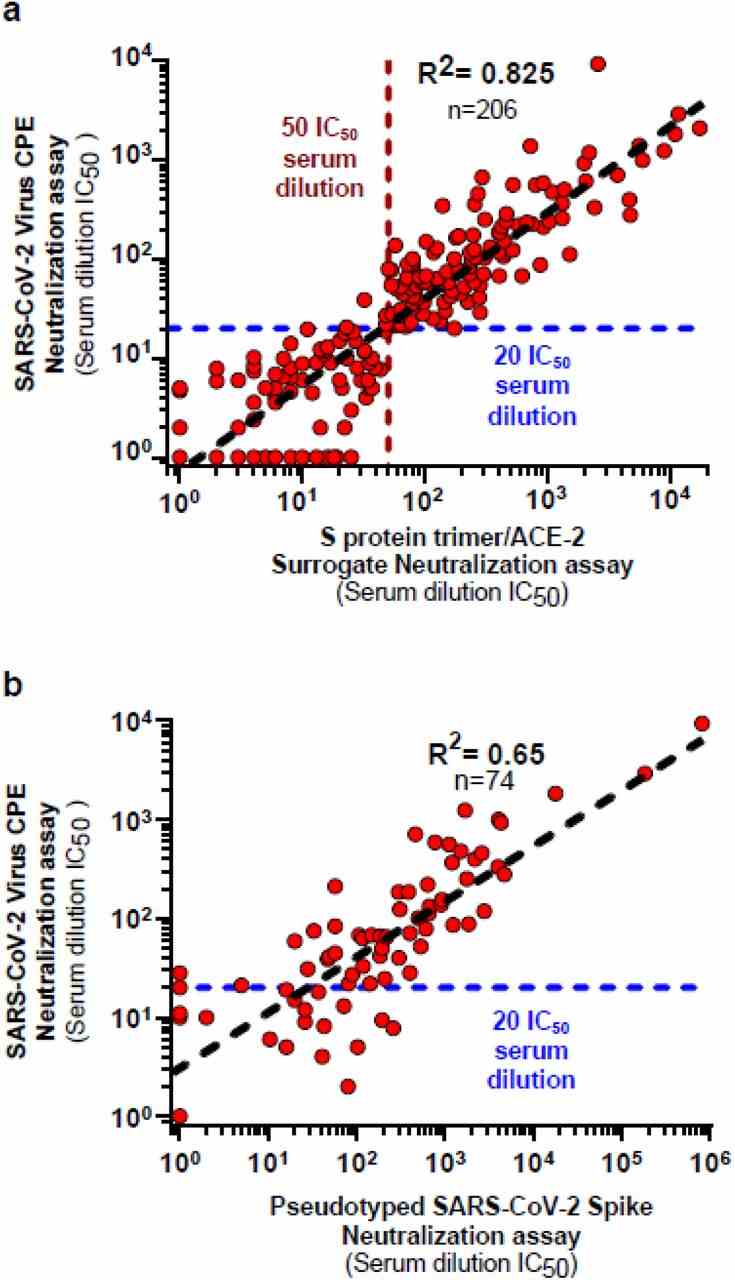
( a ) Cross-validation studies between S 3
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All Ace2 Products
Required fields are marked with *
My Review for All Ace2 Products
Required fields are marked with *



