Ubiquitin-related Molecules in the Akt Pathway
Related Symbol Search List
- cIAP1
- BRCA1
- CBL
- MDM2
- NEDD4
- SKP2
- SMURF2
- STUB1
- TNFAIP3
- TRAF1
- Trim63
- UBA7
- UBA1
- UBE2C
- UBE2F
- UBE2L3
- UBC13
- UBE2N
- UBE2V1
- UCHL1
- UCHL3
- USP8
Immunology Background
About Ubiquitin-related Molecules in the Akt Pathway
The Akt pathway, also known as the PI3K/Akt pathway, is a signaling pathway that plays a crucial role in regulating cell growth, proliferation, survival, and metabolism. It is dysregulated in many diseases, including cancer, diabetes, and neurodegenerative disorders. Ubiquitin-related molecules are important players in the regulation of the Akt pathway.
Ubiquitin is a small protein that can be covalently attached to target proteins in a process called ubiquitination. This modification can have various effects on the target protein, including proteasomal degradation, subcellular localization, protein-protein interactions, and modulation of protein activity. Ubiquitination is a highly dynamic and tightly regulated process that involves the concerted actions of a cascade of enzymes, including ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3).
Several ubiquitin ligases have been identified to play a role in the regulation of the Akt pathway. One example is the E3 ubiquitin ligase called MDM2, which negatively regulates Akt signaling by targeting Akt for proteasomal degradation. MDM2 promotes the ubiquitination and subsequent degradation of Akt, thereby attenuating its activity and downstream signaling.
On the other hand, the deubiquitinating enzyme (DUB) called USP9X has been found to positively regulate the Akt pathway. USP9X stabilizes Akt by removing ubiquitin molecules from it, preventing its degradation. This leads to enhanced Akt activity and signaling.
In addition to ubiquitination and deubiquitination, other ubiquitin-related molecules also play a role in modulating the Akt pathway. For example, the NEDD8 protein (UBE2F), a ubiquitin-like molecule, has been shown to regulate Akt activation. NEDD8 conjugation to specific proteins leads to their activation, which in turn results in Akt activation and downstream signaling.
Furthermore, ubiquitin-like modifiers such as SUMO (small ubiquitin-like modifier) and ISG15 (interferon-stimulated gene 15) have also been identified as important regulators of cellular processes. SUMO is a small protein that can be covalently attached to other proteins, similar to the process of ubiquitination. It plays a role in various cellular processes including protein localization, stability, and activity modulation. SUMOylation has been implicated in DNA repair, transcriptional regulation, and cell cycle progression, among other functions. ISG15, on the other hand, is an interferon-induced protein that is conjugated to target proteins in a process called ISGylation. It has been primarily studied in the context of antiviral immune response, where it functions as a signaling molecule and plays roles in antiviral defense mechanisms. ISG15 has also been implicated in other cellular processes such as protein degradation, inflammation, and DNA damage repair. Both SUMO and ISG15 have emerged as important regulators of cellular pathways, with diverse functions beyond their similarities to ubiquitin.
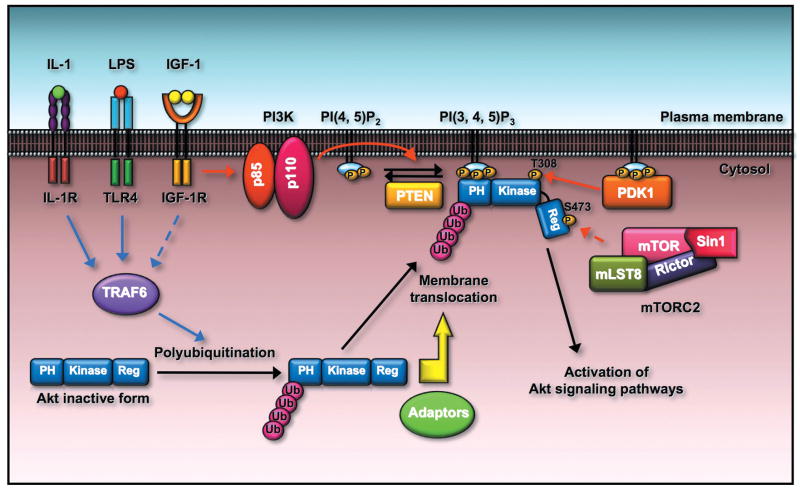 Fig.1 Akt membrane localization and activation is regulated by ubiquitination of Akt. (Yang W L, et al., 2010)
Fig.1 Akt membrane localization and activation is regulated by ubiquitination of Akt. (Yang W L, et al., 2010)
TRAF6 E3 ligase is activated by engagement of IGF-1, IL-1, and LPS to their cognate receptors. The activated TRAF6 then interacts with Akt and triggers K63-linked ubiquitination of Akt, which facilitates Akt membrane recruitment and subsequent phosphorylation by PDK1 and mTORC2 through an unknown mechanism. It is possible that the ubiquitinated Akt may recruit the essential adaptors to facilitate Akt membrane localization.
Understanding the roles and interplay of these ubiquitin-like modifiers is crucial for deciphering the complexity of cellular processes and their regulation.
Mechanism of Action of Ubiquitin-related Molecules in the Akt Pathway
Ubiquitin-related molecules play a key role in regulating the components of the Akt pathway at multiple levels. Below is a description of the mechanism of action of ubiquitin-related molecules at various stages of the Akt pathway:
Ligand-Receptor Interaction: Ubiquitin-related molecules can modulate the binding of ligands to their respective receptors in the Akt pathway. For instance, they can regulate the availability and activity of growth factors, such as insulin-like growth factor 1 (IGF-1) or insulin, by either promoting or inhibiting their binding to the corresponding receptors. This step is crucial for initiating the activation of the Akt pathway.
- The Akt pathway is primarily activated by growth factors such as insulin, insulin-like growth factor 1 (IGF-1), and epidermal growth factor (EGF). The binding of these ligands to their respective receptors (such as insulin receptor and insulin-like growth factor receptor 1) triggers receptor dimerization and autophosphorylation, leading to the recruitment and activation of PI3K (phosphatidylinositol 3-kinase).
- Ubiquitin-related molecules such as the E3 ubiquitin ligases Cbl-b and c-Cbl negatively regulate receptor activation in the Akt pathway. They bind to the activated receptors and target them for ubiquitination and degradation, thereby attenuating the signaling cascade.
Receptor Activation: Ubiquitin-related molecules also participate in the activation of receptors in the Akt pathway. They can either assist in the receptor dimerization required for activation or promote the recruitment of downstream signaling molecules. Additionally, they can regulate the internalization and trafficking of activated receptors, controlling receptor turnover and signaling duration.
- One of the key steps in Akt pathway activation is the phosphorylation of Akt by upstream kinases, such as phosphoinositide-dependent kinase 1 (PDK1) and the mechanistic target of rapamycin complex 2 (mTORC2). Ubiquitin ligases, such as Nedd4-1 and Nedd4-2, have been shown to regulate Akt by promoting its ubiquitination and degradation, thus negatively regulating its activity. These ubiquitin ligases target specific domains of Akt, such as the PH domain, for ubiquitination, leading to its degradation.
- On the other hand, ubiquitin-related molecules, such as deubiquitinases (DUBs), can also regulate Akt signaling by removing the ubiquitin modifications from Akt or its regulators. For example, USP9X, a DUB, deubiquitinates and stabilizes PTEN (phosphatase and tensin homolog), a negative regulator of Akt signaling. By stabilizing PTEN, USP9X indirectly inhibits Akt signaling.
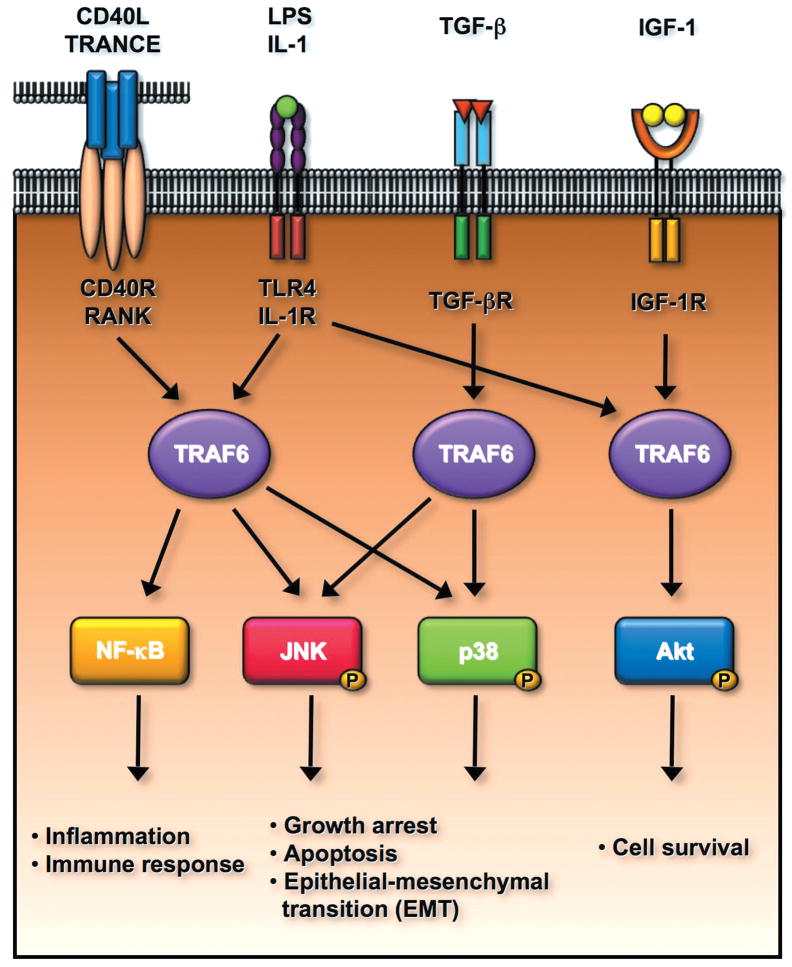 Fig.2 TRAF6 E3 ligase regulates multiple signal transduction pathways involved in the plethora of biological functions including the innate immune response, cell survival, apoptosis, and epithelial-mesenchymal transition (EMT). (Yang W L, et al., 2010)
Fig.2 TRAF6 E3 ligase regulates multiple signal transduction pathways involved in the plethora of biological functions including the innate immune response, cell survival, apoptosis, and epithelial-mesenchymal transition (EMT). (Yang W L, et al., 2010)
TRAF6 is a critical mediator for NF-κB activation upon the engagement of CD40L, TRANCE, LPS and IL-1 to their receptors, in turn regulating inflammation and innate immune response. TRAF6 is activated by TGF-β/TGF-β receptor and is required for TGF-β-mediated p38 and JNK activation. The activated p38 and JNK positively regulate cell apoptosis and EMT. Interestingly, TRAF6 is also activated upon the binding of IGF-1 to IGF-1 receptor and regulates cell survival by inducing Akt activation.
Downstream Signaling: Once activated, the Akt pathway propagates the signal through a series of downstream signaling molecules. Ubiquitin-related molecules are involved in modulating the activation and activity of these intermediates. They can regulate the phosphorylation of downstream signaling proteins or facilitate the formation of signaling complexes through protein-protein interactions. By doing so, they control the magnitude and duration of Akt pathway signaling.- In the context of the Akt pathway, ubiquitin-related molecules have been shown to regulate the degradation and activity of key components of this pathway. For example, the E3 ubiquitin ligase SCFβ-TrCP has been implicated in the ubiquitination and subsequent degradation of the Akt inhibitor, PTEN. This allows for sustained Akt activation and downstream signaling.
- Additionally, ubiquitin ligases such as MDM2 and Nedd4-1 have been shown to interact with and regulate the stability of various Akt pathway components, including Akt itself. These ubiquitin ligases can target Akt for degradation or modify its activity through the attachment of ubiquitin chains.
- Furthermore, ubiquitin-related molecules have been implicated in the regulation of downstream effectors of Akt signaling. For instance, the activation of Akt can lead to the phosphorylation and inactivation of the forkhead box O (FOXO) transcription factors, which regulate the expression of genes involved in cell cycle arrest and apoptosis. Ubiquitin ligases, such as MDM2 and β-TrCP, have been shown to mediate the degradation of FOXO proteins, thus attenuating their functions in response to Akt activation.
Pathway Termination: Ubiquitin-related molecules also have a role in attenuating the Akt pathway signaling. They facilitate the ubiquitination and subsequent proteasomal degradation of key signaling components, including receptors, signaling intermediates, and negative regulators. This degradation ensures pathway termination and prevents prolonged signaling, which could lead to aberrant cell proliferation or survival.
- For example, the E3 ubiquitin ligase named SCF (Skp, Cullin, F-box protein)-β-TRCP plays a crucial role in terminating the Akt pathway. SCF-β-TRCP recognizes and binds to phosphorylated Akt, resulting in the ubiquitination and subsequent degradation of Akt by the proteasome. This degradation process leads to the termination of Akt signaling, preventing sustained activation of downstream targets.
- Moreover, other ubiquitin-related molecules, such as deubiquitinating enzymes (DUBs), also participate in regulating the Akt pathway termination. DUBs can remove ubiquitin from target proteins, thereby rescuing them from degradation or modulating their activity. DUBs can also regulate the turnover of E3 ubiquitin ligases, influencing their ability to terminate Akt signaling.
In summary, ubiquitin-related molecules are involved in multiple stages of the Akt pathway, playing a crucial role in regulating ligand-receptor interaction, receptor activation, downstream signaling, and pathway termination. Their involvement ensures proper signal transduction and prevents excessive or prolonged signaling, ultimately maintaining cellular homeostasis.
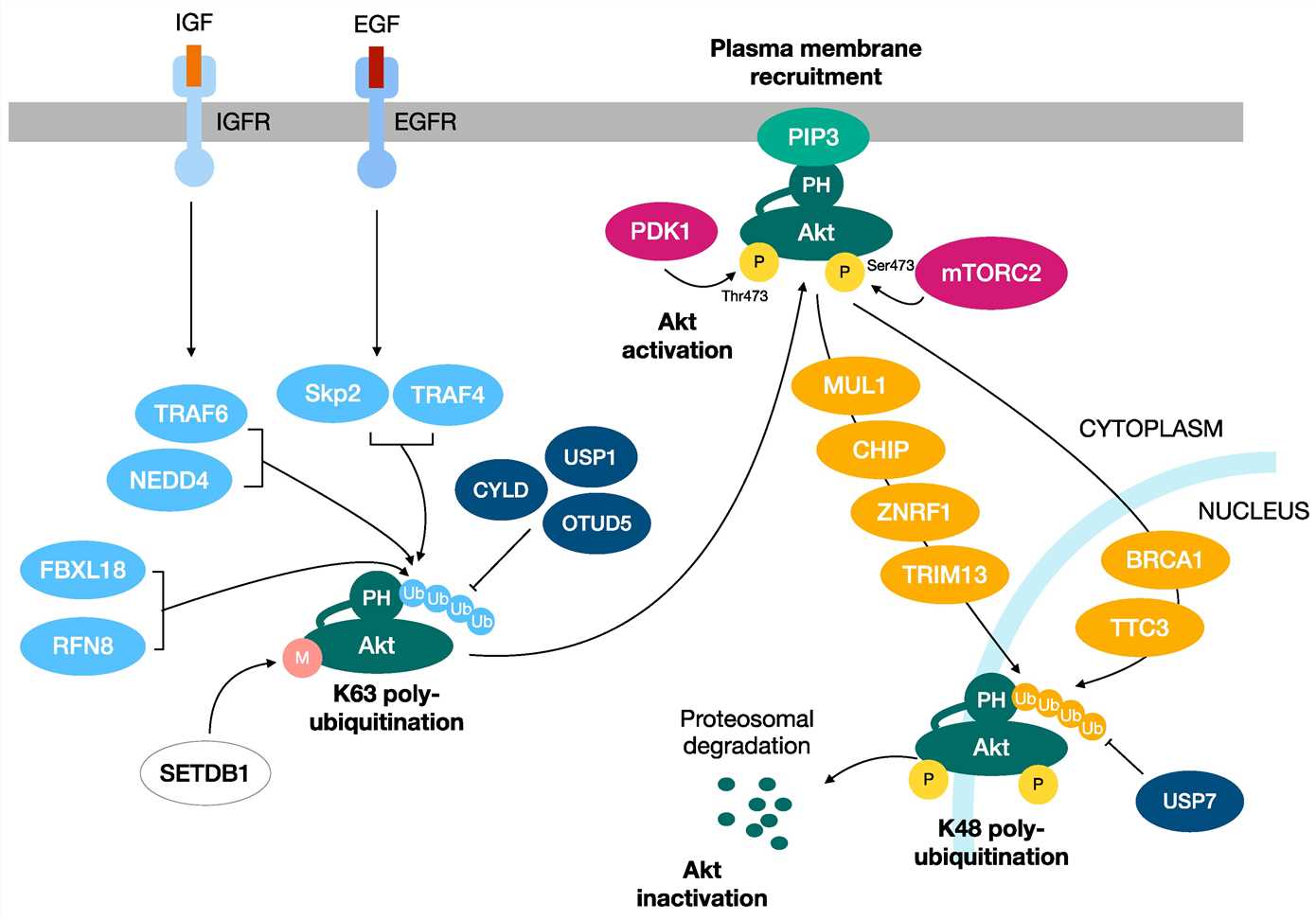 Fig.3 Two distinct ubiquitination processes regulate the activation and inactivation of Akt kinase. (Paccosi E, et al., 2023)
Fig.3 Two distinct ubiquitination processes regulate the activation and inactivation of Akt kinase. (Paccosi E, et al., 2023)
K48-linked ubiquitination promotes the proteasomal degradation of Akt, turning off its activity and downstream responses. Instead, the K63-linked polyubiquitination of Akt has a role in the activation of Akt, functioning as a regulatory signal that induces the plasma membrane recruitment, serine/threonine phosphorylation and activation of Akt.
Available Resources for Ubiquitin-related Molecules in the Akt Pathway
Creative BioMart offers a variety of research tools related to ubiquitin, aiding researchers in delving into the regulatory mechanisms of the Akt pathway. These research tools include recombinant proteins, cell and tissue lysates, protein pre-coupled beads, and custom services. Through the utilization of these tools, researchers can investigate ubiquitin-related molecules within the Akt pathway, gaining deeper insights into their functions and interactions within this pathway.
The following are the molecules/targets of ubiquitin-related molecules in the Akt pathway. Click to view product details.
In addition to providing research tools, Creative BioMart also offers a wide range of technical and educational resources to assist researchers in better understanding and applying these tools. We integrate these tools and resources for easy access and retrieval of relevant information, including literature, technical guides, and operation protocols, enabling researchers to better utilize these research tools for scientific inquiry.
Contact us today to learn how our products and customized services can advance your research into the ubiquitin-related molecules in the Akt pathway.
References:
- Yang W L, Wu C Y, Wu J, et al. Regulation of Akt signaling activation by ubiquitination[J]. Cell Cycle, 2010, 9(3): 486-497.
- Paccosi E, Balzerano A, Proietti-De-Santis L. Interfering with the Ubiquitin-Mediated Regulation of Akt as a Strategy for Cancer Treatment[J]. International Journal of Molecular Sciences, 2023, 24(3): 2809.

