JUN
-
Official Full Name
jun proto-oncogene -
Overview
Transcription factor AP-1; JUN; AP-1; AP1; c-Jun; jun proto-oncogene; p39; jun oncogene; activator protein 1; proto-oncogene c-Jun; enhancer-binding protein AP1; Jun activation domain binding protein; v-jun sarcoma virus 17 oncogene homolog; v-jun avian sarcoma virus 17 oncogene homolog -
Synonyms
JUN;jun proto-oncogene;jun oncogene ,v jun avian sarcoma virus 17 oncogene homolog ,v jun sarcoma virus 17 oncogene homolog (avian);transcription factor AP-1;AP 1;c Jun;Activator Protein 1;AP 1;AP1;cJun;Enhancer Binding Protein AP1;Jun Activation Domain Binding Protein;JUN;Jun oncogene;JUN protein;Jun proto oncogene;JUN_HUMAN;JUNC;Oncogene JUN;p39;Proto oncogene c jun;Proto oncogene cJun;Proto-oncogene c-jun;Transcription Factor AP 1;Transcription factor AP-1;Transcription Factor AP1;V jun avian sarcoma virus 17 oncogene homolog;V jun sarcoma virus 17 oncogene homolog (avian);V-jun avian sarcoma virus 17 oncogene homolog;vJun Avian Sarcoma Virus 17 Oncogene Homolog;p39;jun oncogene;OTTHUMP00000010036;activator protein 1;proto-oncogene c-Jun;enhancer-binding protein AP1;Jun activation domain binding protein;v-jun sarcoma virus 17 oncogene homolog;v-jun avian sarcoma virus 17 oncogene homolog;AP1;AP-1;c-Jun
Recombinant Proteins
- Human
- Zebrafish
- Rhesus macaque
- Chicken
- Rat
- Mouse
- Insect Cells
- E.coli
- Mammalian Cells
- HEK293
- HeLa
- In Vitro Cell Free System
- Non
- His
- MBP
- GST
- Avi
- Fc
- Myc
- SUMO
Background
What is JUN protein?
JUN (Jun proto-oncogene, AP-1 transcription factor subunit) gene is a protein coding gene which situated on the short arm of chromosome 1 at locus 1p32. c-Jun is an important proto-oncogene and transactivating factor that is a component of the AP-1 and ATF-2 transcription factors. c-Jun is acutely regulated by a wide variety of cellular signals via modulation of its phosphorylation state, which can both inhibit and activate the proto-oncogene. The JUN protein is consisted of 331 amino acids and its molecular mass is approximately 35.7 kDa.
What is the function of JUN protein?
JUN, also known as c-JUN, is a basic leucine zipper transcription factor that is a key component of the AP-1 (activator protein-1) transcription complex. c-Jun plays a key role in a variety of biological processes, including cell proliferation, differentiation, apoptosis and stress response. It regulates the expression of many genes by binding to specific DNA sequences, thereby affecting the fate and function of cells.
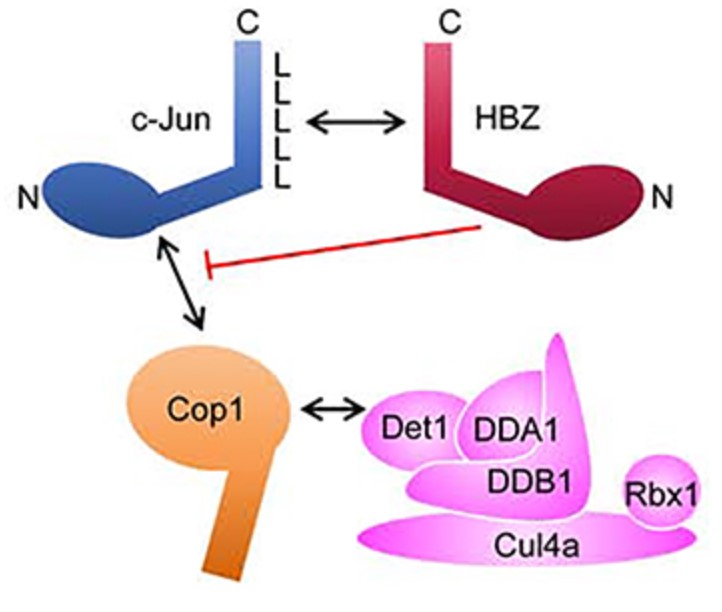
Fig1. Schematic representation of c-Jun degradation initiated by Cop1. (Nicholas Polakowski, 2020)
JUN Related Signaling Pathway
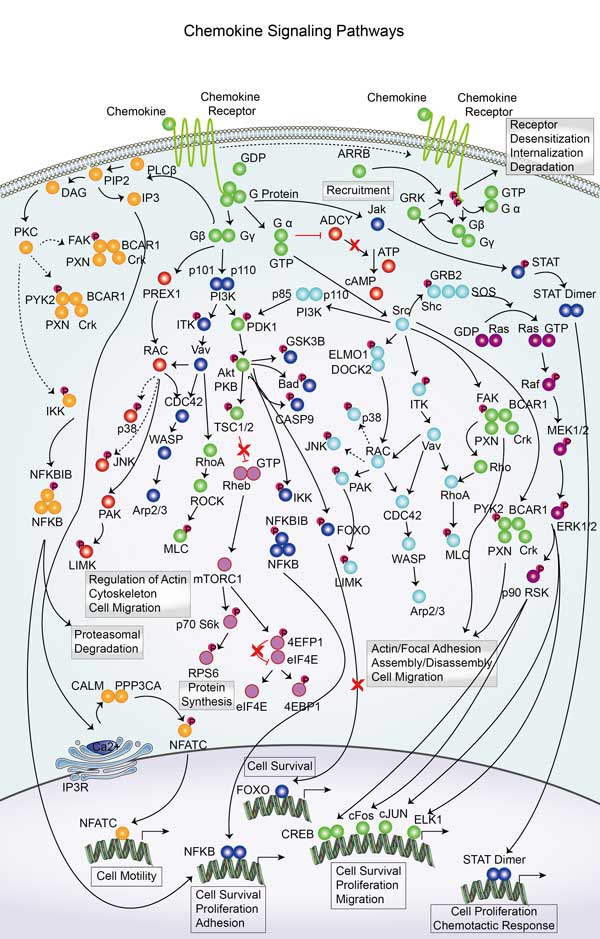
JUN is associated with many signaling pathways, including MAPK/ERK, JNK and p38. These pathways play an important role in cellular stress response, inflammatory response and tumorigenesis. By binding to specific DNA sequences, c-Jun can regulate the expression of many genes, thereby affecting the fate and function of cells.
JUN Related Diseases
c-Jun has been found to be over-activated in many types of cancer, including lung, breast, colon and skin cancers. Second, c-Jun has also been linked to neurodegenerative diseases such as Alzheimer's and Parkinson's disease. In these diseases, overactivation of c-Jun may lead to damage and death of nerve cells. In addition, c-Jun is also associated with cardiovascular diseases, inflammatory diseases and autoimmune diseases.
Bioapplications of JUN
Because c-Jun is overactive in many types of cancer, it is seen as a potential drug target. By developing small molecule drugs capable of inhibiting c-Jun activity or using RNA interference techniques, new strategies for cancer treatment may be available. In addition, c-Jun expression levels have also been used to assess prognosis and disease progression in certain cancers. For example, in some types of lung and breast cancer, high expression of c-Jun is associated with worsening of the disease and poor prognosis.
Case Study
Case study 1: Nicholas Polakowski, 2020
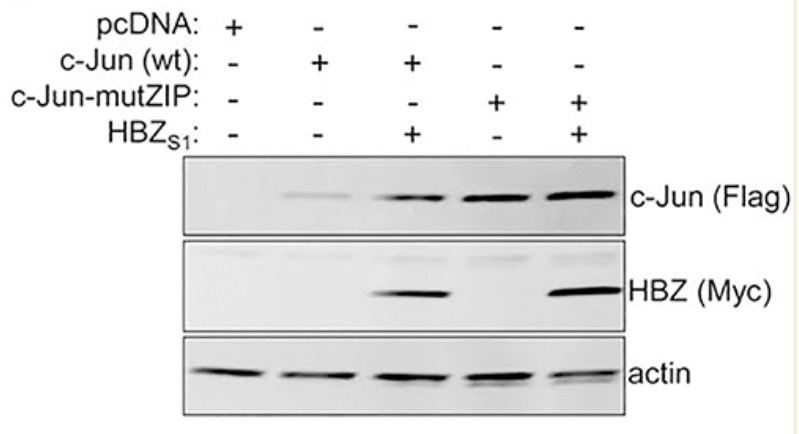
HBZ is expressed by the complex retrovirus, Human T-cell Leukemia Virus type 1, and implicated in pathological effects associated with viral infection. From the nucleus, HBZ alters gene expression by interacting with a variety of transcriptional regulatory proteins, among which is c-Jun. Previously, one of the three HBZ variants, HBZUS, was reported to decrease c-Jun expression by promoting its degradation. Here the researchers show that another variant, HBZS1, produces the opposite effect. In the presence of HBZS1, c-Jun expression increases due to its stabilization. Our data suggest that this effect requires the ability of HBZS1 to interact with c-Jun. They provide evidence that HBZS1 inhibits the proteosomal degradation of c-Jun initiated by the Cop1-containing ubiquitin ligase complex. HBZS1 is the most abundant variant in HTLV-1-infected T-cells, and the data indicate that levels of c-Jun expression in infected cells are consistent with effects of HBZS1.
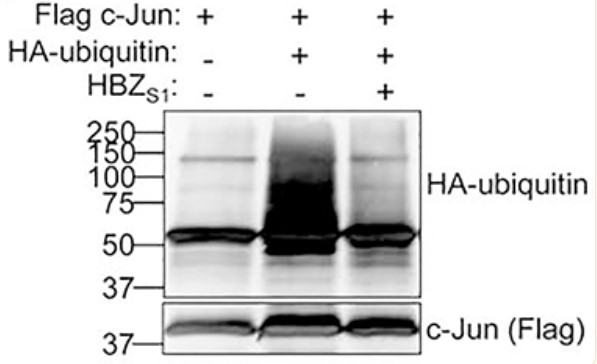
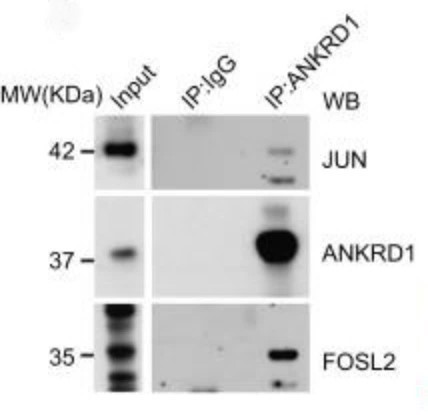
Fig1. Ectopically-expressed c-Jun was immunoprecipitated from MG132-treated HEK293T cells transfected with expression vectors for the indicated plasmids.
Case study 2: Luigi Mazzeo, 2024
There are significant commonalities among several pathologies involving fibroblasts, ranging from auto-immune diseases to fibrosis and cancer. Early steps in cancer development and progression are closely linked to fibroblast senescence and transformation into tumor-promoting cancer-associated fibroblasts (CAFs), suppressed by the androgen receptor (AR). Here, the researchers identify ANKRD1 as a mesenchymal-specific transcriptional coregulator under direct AR negative control in human dermal fibroblasts (HDFs) and a key driver of CAF conversion, independent of cellular senescence. ANKRD1 expression in CAFs is associated with poor survival in HNSCC, lung, and cervical SCC patients, and controls a specific gene expression program of myofibroblast CAFs (my-CAFs). ANKRD1 binds to the regulatory region of my-CAF effector genes in concert with AP-1 transcription factors, and promotes c-JUN and FOS association. Targeting ANKRD1 disrupts AP-1 complex formation, reverses CAF activation, and blocks the pro-tumorigenic properties of CAFs in an orthotopic skin cancer model. ANKRD1 thus represents a target for fibroblast-directed therapy in cancer and potentially beyond.
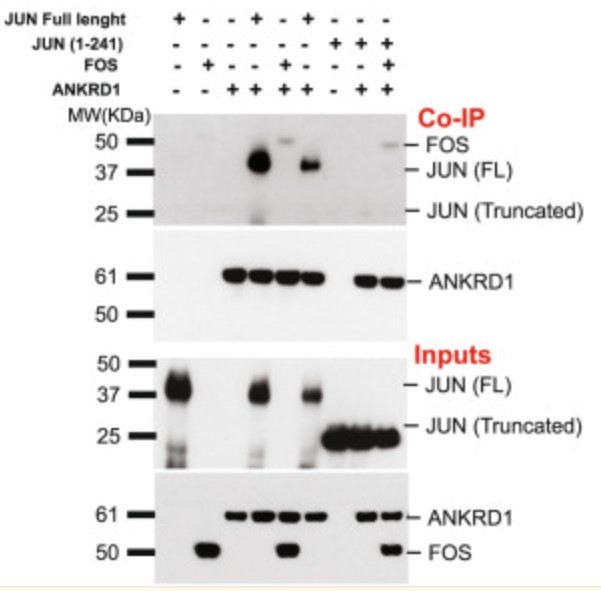
Fig3. In vitro protein interactions. Glutathione-conjugated beads were used to immunoprecipitate GST-tagged ANKRD1 (100 ng) recombinant protein mixed with the following recombinant proteins.
Quality Guarantee
High Purity
Fig1. SDS-PAGE (JUN-18H) (PROTOCOL for western blot)
.

Fig2. SDS-PAGE (JUN-3133H) (PROTOCOL for western blot)
Involved Pathway
JUN involved in several pathways and played different roles in them. We selected most pathways JUN participated on our site, such as AGE/RAGE pathway,ATF-2 transcription factor network,Activated TLR4 signalling, which may be useful for your reference. Also, other proteins which involved in the same pathway with JUN were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|---|
| Androgen receptor signaling pathway | RNF6,PSMC3IP,AES,SMAD3B,RAD9A,KAT7B,MYST2,POU2F1B,DSTN,TGFB1I1 |
| ATF-2 transcription factor network | POU2F1,ATF2,DUSP10,KCNA3,KAT5,RUVBL2,MAPK14,JUND,JDP2,DDIT3 |
| Apoptosis | STK24A,IL1B2,DIABLO,PLECA,Fasl,CASP7,PRKACA,RELA,DNM1L,CFLARA |
| Activated TLR4 signalling | TANK,TICAM1,PELI1,CHUK,PELI2,TIRAP,TAB1,TLR6,DUSP7,ATF2 |
| AhR pathway | CYP1A2,CDKN1B,NQO1,AHR,CDKN1A,CDC37,CYP1B1,FLG,JUND,PTGES3 |
| AGE/RAGE pathway | AGER,CHUK,EGFR,LGALS3,DIAPH1,DDOST,JAK2,MMP13,MAPK14,TIRAP |
| Activation of the AP-1 family of transcription factors | MAPK14,ATF2 |
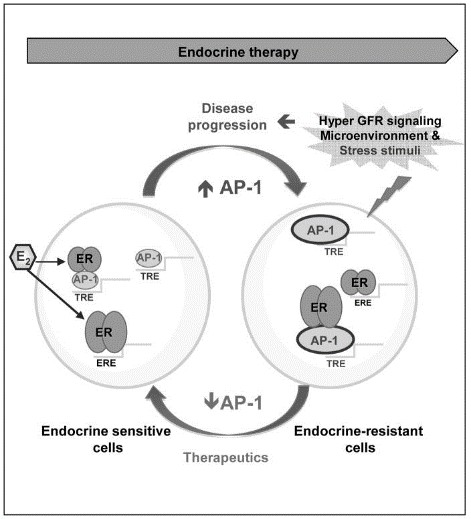
Fig1. Working model: in endocrine-sensitive cells grown in the presence of E2 (left side), ER acts predominantly through classical genomic functions where it binds to DNA at E2 responsive elements (ERE) to modulate gene expression and to convey mitogenic and survival signals. (Luca Malorni, 2016)
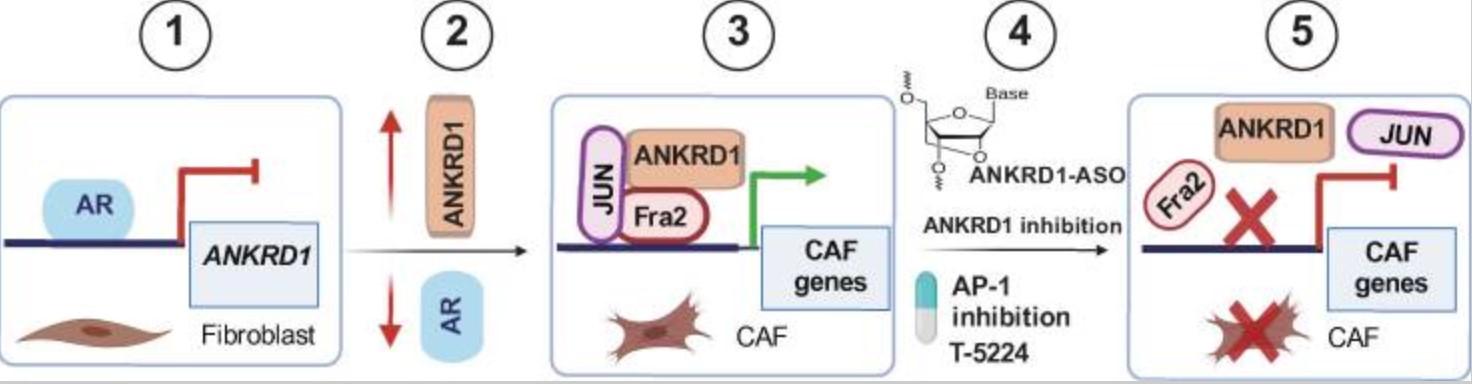
Fig2. Schematic representation of the role of ANKRD1 in CAF activation and potential translational relevance. Fig8. Schematic representation of the role of ANKRD1 in CAF activation and potential translational
Protein Function
JUN has several biochemical functions, for example, DNA binding,GTPase activator activity,HMG box domain binding. Some of the functions are cooperated with other proteins, some of the functions could acted by JUN itself. We selected most functions JUN had, and list some proteins which have the same functions with JUN. You can find most of the proteins on our site.
| Function | Related Protein |
|---|---|
| RNA polymerase II distal enhancer sequence-specific DNA binding | REL,MED12,RELA,NR5A1,NR2F6,ZBTB16,FLI1,BACH2,T,RXRB |
| poly(A) RNA binding | NUPL2,TBL3,PEG10,SRSF2,RPL28,MKI67IP,AKAP8L,RPGR,ARL6IP4,RPL11 |
| GTPase activator activity | ELMOD1,SOS1,ARHGAP33,TBC1D9B,SGSM3,GAPVD1,ARHGAP1,RGS5B,LLGL2,ALDH1A2 |
| transcription coactivator activity | BIRC2,GATA3,NR2C2,RNF4,FHL2,RARA,PPARGC1A,RFXAP,TADA2A,TCERG1 |
| transcription factor activity, sequence-specific DNA binding | HOXB6B,MYCN,POU1F1,NFIXB,ATF7B,TBX21,HSF5,RBPJ,NFIL3-4,EVX1 |
| transcription factor activity, RNA polymerase II distal enhancer sequence-specific binding | TEAD2,USF2,TLE4,POU4F1,MYF6,SRY,MYOD1,FOXF1,NKX2-5,SOX10 |
| R-SMAD binding | FOXH1,ZC3H3,RANBP3,FOS,PPM1A,PARP1,SMURF1,SMAD2,ZEB2,C18orf1 |
| protein binding | TOMM34,RFX6,RBM3,SMOC1,RNF175,SSNA1,ZNF34,SYT17,ATRIP,GCA |
| transcriptional activator activity, RNA polymerase II transcription factor binding | CREB1,LMO2,KLF14,CLOCK,WWP2,NKX2,NFE2L1,MIXL1,NR1H4,PITX1 |
Interacting Protein
JUN has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with JUN here. Most of them are supplied by our site. Hope this information will be useful for your research of JUN.
FOS
JUN Related Signal Pathway
Resources
Research Area
- Transcription Factors in the Akt Pathway
- Negative Regulators of the Jak/STAT Pathway
- Positive Regulators of the Jak/STAT Pathway
- Cancer Stem Cell Transcription Factors
- Hematopoietic Stem Cell Transcription Factors and Regulators
- Mesenchymal Stem Cell Transcription Factors
- Neural Stem Cell Transcription Factors
- Wnt Intracellular Signaling
- Pluripotent Stem Cell Transcription Factors
- C-type Lectin Receptors
- NOD-like Receptors and the Inflammasome
- IL-17 Signaling Related Molecules
- Oncoprotein-Transcription Factors
Related Services
Related Products
References
- Toyota, H; Sudo, K; et al. Thy28 protects against anti-CD3-mediated thymic cell death in vivo. APOPTOSIS 20:444-454(2015).
- Rodriguez, AM; Davila, MP; et al. Tumor Necrosis Factor alpha Phosphorylates c-Jun N-Terminal Kinase in Stallion Spermatozoa: Effect of Cryopreservation. JOURNAL OF EQUINE VETERINARY SCIENCE 35:206-212(2015).