Fucose-free Protein Expression System
Unlock the next generation of therapeutic proteins with our Fucose-Free Protein Expression System . At Creative BioMart , we leverage our proprietary Fut8-/- CHO-K1 cell line to produce high-activity, low-cytotoxicity proteins without core fucosylation. This innovative glycoengineering approach enhances therapeutic efficacy—particularly in oncology—and reduces production costs, side effects, and immunogenic risk. Whether you're advancing a novel biologic or biosimilar, our system delivers proteins with optimized glycosylation profiles tailored for therapeutic success.
Background: Why Fucose-Free Proteins Matter in Biopharmaceutical Development
Fucose (C6H12O5) is a hexose deoxy sugar frequently found in N -linked glycans on the surface of mammalian, insect, and plant cells. In humans, it is typically attached via an α-1,6 linkage to the core N -acetylglucosamine (GlcNAc) residue of N -linked glycans. Fucose may also contribute to the structure of blood group antigens or serve as a terminal sugar in glycan branches.
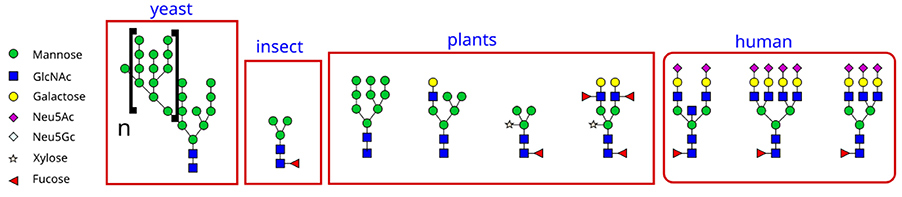
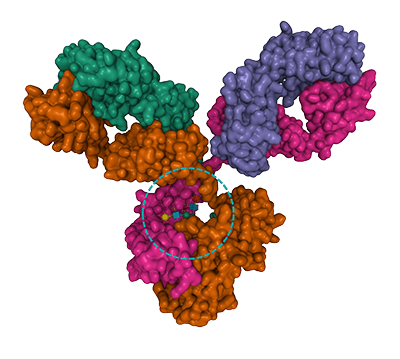
Figure 1. Fucose is found on N- linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan.
In the context of biologics, fucosylated glycans can significantly affect a protein’s pharmacokinetics, immunogenicity, and functional activity. Core fucosylation often reduces antibody-dependent cellular cytotoxicity (ADCC)—a critical mechanism of action for many anticancer antibodies. Hence, removing core fucose can dramatically improve efficacy.
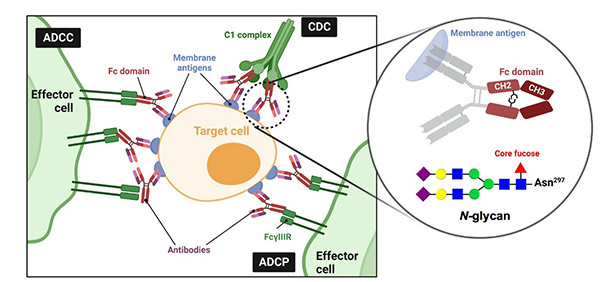
Figure 2. Antibody fucosylation and existing metabolic inhibitors of fucosylation. (Gilormini et al ., 2024)
To address these challenges, Creative BioMart has developed a Fut8 -knockout ( Fut8-/- ) CHO-K1 cell line. This proprietary cell line lacks the fucosyltransferase 8 (Fut8) gene, effectively eliminating core fucosylation during recombinant protein expression. The result is a production platform capable of generating fucose-free therapeutic proteins with enhanced potency, reduced immunotoxicity, and improved clinical performance.
Service Overview: Fucose-Free Protein Expression System
Service Procedure
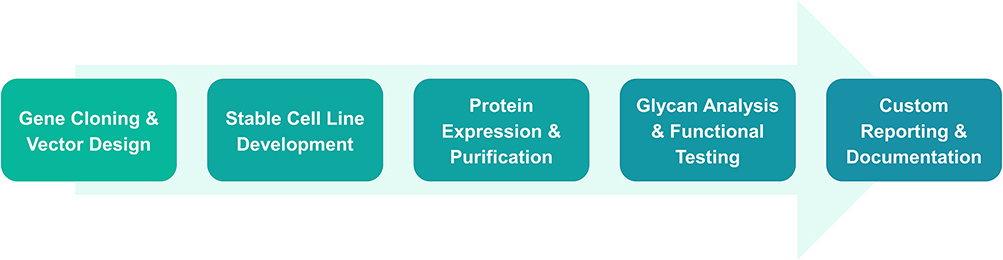
Service Details
-
Fut8-/- CHO-K1 cell line (stable or transient expression)
-
Antibodies , enzymes, fusion proteins, cytokines , and more
-
≥95% by SDS-PAGE and HPLC
-
mg to gram scale, scalable to industrial levels
-
Glycan mapping, SEC, ELISA, bioactivity assays
-
Codon optimization , tag selection, vector backbone flexibility
Service Advantages: Why Choose Our Fucose-Free Protein Expression System
- Enhanced ADCC Activity : Eliminating core fucose boosts the therapeutic potency of monoclonal antibodies—especially in cancer immunotherapies—by improving their ability to recruit immune cells.
- Lower Cytotoxicity : Proteins produced in our Fut8-/- CHO-K1 cells show reduced off-target effects, leading to safer biologics.
- Reduced Production Costs : Our streamlined cell line reduces glycosylation heterogeneity, improving yield and simplifying downstream processing.
- Decreased Immunogenicity : Avoiding allergenic α-1,3 fucosylation helps minimize IgE-mediated immune responses.
- Proven Platform : Our CHO-K1 Fut8-/- system is industry-tested and optimized for biosimilar and novel biologic development.
- Customizable & Scalable : From early discovery to clinical-scale manufacturing, our services adapt to your project’s scope and timeline.
Case Studies: Real-World Results with Fucose-Free Protein Expression System
* NOTE: We prioritize confidentiality to safeguard our clients’ technology and intellectual property. As an alternative, we present selected published research articles as representative case studies. For details on the assay services and products used in these studies, please refer to the relevant sections of the cited literature.
Case 1: Stable antibody expression in FUT8 -KO CHO cells
Gao et al. , 2023. doi:10.21203/rs.3.rs-3691208/v1
Afucosylated antibodies, known for their enhanced antibody-dependent cellular cytotoxicity (ADCC), are gaining traction in clinical development. However, standard Chinese hamster ovary (CHO) cells naturally produce highly fucosylated antibodies, limiting ADCC effectiveness. To address this, researchers used CRISPR/Cas9 to knock out key fucosylation genes— FUT8 , GMD, and FX—in CHO cells. The modified cell lines were then assessed for growth, antibody yield, glycosylation profiles, and ADCC activity. Remarkably, the knockout cells maintained normal morphology and growth, while producing completely afucosylated antibodies. In particular, FUT8 knockout ( FUT8KO ) cells showed a 2–2.5-fold increase in ADCC activity over conventional CHO cells. Specific FUT8KO monoclonal clones demonstrated high stability and productivity, making them excellent candidates for therapeutic antibody production with superior clinical potential.
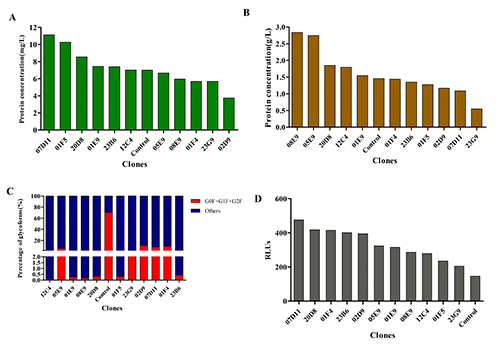
Figure 3. Antibody titers, N-glycan profiles, and ADCC activity of the cells after fed-batch culture. (A). Afucosylated antibody titers on 48 h post-transfection of FUT8KO monoclonal cell lines using ELISA assay. (B). Afucosylated antibody titers at the end of fed-batch culture of FUT8KO monoclonal cell lines using the Octet QK system. (C). The N-glycans analysis results of the FUT8KO monoclonal cell lines. (D) The relative ADCC activity FUT8KO monoclonal cell lines. (Gao et al ., 2023)
Case 2: CHO cell glycosylation mutants to advance glycobiology
Haryadi et al ., 2024. doi:10.1038/s41598-024-73722-z
This study presents a comprehensive panel of glycosylation mutant CHO cell lines, designed to advance glycobiology and therapeutic protein engineering. Two model proteins, EPO-Fc and trastuzumab, were expressed in these mutants to analyze the impact of specific glycogene knockouts on glycan structures, using LC-MS/MS. EPO-Fc with homogeneous Man9 glycans was only achieved by inactivating nearly all α-mannosidases, except MAN2B1. Mutants lacking GnT-I activity produced predominantly Man5 N-glycans, while those missing GnT-II altered branching patterns, yielding single-branched glycans with higher sialic acid content than standard CHO-K1 dual-branched glycans. Trastuzumab expressed in these cells revealed how altered Fc N-glycans affect binding to Fcγ receptors (FcγRI, FcγRIIa, and FcγRIIIa), influencing ADCC. Additionally, trimeric HIV gp120 produced in mutants showed varied reactivity with broadly neutralizing antibodies, highlighting glycan involvement in antibody recognition. One mutant also produced β-glucocerebrosidase with uniform Man5 glycans, demonstrating potential for improved therapeutic glycoprotein production.
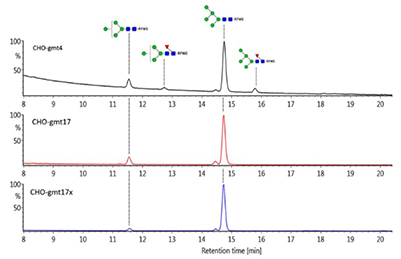
Figure 4. Recombinant b-glucocerebrosidase (GCase) from CHO-gmt4, CHO-gmt17 and CHO-17x was purified and analyzed by LC-MS. The Y-axis shows the relative fluorescence intensity, with the highest peak as 100%. (Haryadi et al . , 2024)
What Our Clients Say About Our Fucose-Free Protein Expression Services
“We partnered with Creative BioMart to produce a fucose-free version of our anti-HER2 monoclonal antibody using their Fut8-/- CHO-K1 platform. The results exceeded expectations—our in vitro ADCC assays showed over a 5-fold increase in activity compared to the fucosylated version. The turnaround was quick, documentation was thorough, and their team was responsive throughout.”
— Director of Biologics R&D | Mid-Sized Oncology Biotech (U.S.)
“Our IL-15 Fc-fusion protein project required a fucose-free expression system to enhance immune stimulation while reducing off-target effects. Creative BioMart delivered a highly pure afucosylated product that retained structural integrity and bioactivity. The analytical support—especially glycan profiling—was meticulous.”
— Senior Scientist, Immunotherapy Division | Global Pharma Company (Europe)
“We were developing a novel afucosylated GM-CSF variant for preclinical autoimmune disease models. Creative BioMart not only provided expression and purification services, but also helped interpret glycan analysis and recommended key adjustments to improve stability. Their scientific insight was invaluable, not just their technical capabilities.”
— Principal Investigator | Academic Translational Research Lab (Canada)
“We needed a stable cell line for producing a biosimilar to a known afucosylated therapeutic antibody. Creative BioMart’s Fut8-/- CHO-K1 system provided high yield (~180 mg/L) and consistent glycosylation across batches. They handled codon optimization and purification seamlessly. Excellent value and consistent quality.”
— Group Leader, Protein Sciences | Biosimilar Manufacturing Firm (India)
Frequently Asked Questions: Fucose-Free Protein Expression
-
Q: What is a fucose-free protein, and why does it matter?
A: Fucose-free proteins lack core fucosylation in their glycans. This enhances ADCC activity, improving therapeutic potency—especially in antibodies—while reducing side effects and dosage needs. -
Q: What system do you use to produce fucose-free proteins?
A: We use a proprietary Fut8-/- CHO-K1 cell line, engineered to eliminate core fucose from N-linked glycans. It’s ideal for biologics requiring high efficacy and low immunogenicity. -
Q: What protein types can you express?
A: We support a range of proteins, including:- Monoclonal antibodies
- Fc-fusion proteins
- Cytokines and enzymes
Perfect for both novel and biosimilar therapeutics.
-
Q: Do you confirm the proteins are truly fucose-free?
A: Yes. We perform glycan profiling and can provide functional assays like ADCC and binding tests to confirm performance. -
Q: How are your yields and purity levels?
A: We deliver high yields (mg–g scale) and ≥95% purity, while maintaining consistent afucosylation and low cytotoxicity.
Resources
Related Services
- Stable Cell Line Services
- GMP Stable Cell Line Development Platform
- Recombinant Antibody Production Services
- Fc Fusion Protein Production
- Albumin Fusion Protein Production Services
- High Yield Protein Production
- Protein Analytical Service
- Codon Optimization
- SUMO-tag Protein Production
Related Products
References:
- Gao L, Zhang M, Li B, et al . Establishment of FUT8 gene knockout CHO cell line with stable expression of monoclonal antibody. Published online December 7, 2023. doi:10.21203/rs.3.rs-3691208/v1
- Gilormini PA, Thota VN, Fers-Lidou A, et al . A metabolic inhibitor blocks cellular fucosylation and enables production of afucosylated antibodies. Proc Natl Acad Sci USA . 2024;121(27):e2314026121. doi:10.1073/pnas.2314026121
- Haryadi R, Chan KF, Lin PC, et al . Generating and characterizing a comprehensive panel of CHO cells glycosylation mutants for advancing glycobiology and biotechnology research. Sci Rep . 2024;14(1):23068. doi:10.1038/s41598-024-73722-z
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

