Recombinant Mouse BTRC Protein
| Cat.No. : | BTRC-2545M |
| Product Overview : | Recombinant Mouse BTRC full length or partial length protein was expressed. |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Mouse |
| Source : | Mammalian Cells |
| Tag : | His |
| Form : | Liquid or lyophilized powder |
| Endotoxin : | < 1.0 EU per μg of the protein as determined by the LAL method. |
| Purity : | >80% |
| Notes : | This item requires custom production and lead time is between 5-9 weeks. We can custom produce according to your specifications. |
| Storage : | Store it at +4 ºC for short term. For long term storage, store it at -20 ºC~-80 ºC. |
| Storage Buffer : | PBS buffer |
| Publications : |
| Gene Name | Btrc beta-transducin repeat containing protein [ Mus musculus ] |
| Official Symbol | BTRC |
| Gene ID | 12234 |
| mRNA Refseq | NM_001037758.2 |
| Protein Refseq | NP_001032847.2 |
| MIM | |
| UniProt ID | Q3ULA2 |
| ◆ Recombinant Proteins | ||
| BTRC-399H | Recombinant Human BTRC Protein, GST-tagged | +Inquiry |
| BTRC-1687HF | Recombinant Full Length Human BTRC Protein, GST-tagged | +Inquiry |
| BTRC-99C | Recombinant Cynomolgus Monkey BTRC Protein, His (Fc)-Avi-tagged | +Inquiry |
| BTRC-351C | Recombinant Cynomolgus BTRC Protein, His-tagged | +Inquiry |
| BTRC-2545M | Recombinant Mouse BTRC Protein | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| BTRC-8384HCL | Recombinant Human BTRC 293 Cell Lysate | +Inquiry |
Wnt-Induced Stabilization of KDM4C Is Required for Wnt/β-Catenin Target Gene Expression and Glioblastoma Tumorigenesis
Journal: Cancer research PubMed ID: 31888886 Data: 2021/3/1
Authors: Yaohui Chen, Runping Fang, Suyun Huang
Article Snippet:Empty anti-Flag agarose beads were used as a control.Empty anti-Flag agarose beads were used as a control.. The in vitro ubiquitination assay was performed by incubating KDM4C or control agarose beads at 37°C for 1 hour with E1 ubiquitin-activating enzyme UBE1, E2-conjugating enzyme UbcH5c, HA-ubiquitin, and adenosine triphosphate (all from Boston Biochem) in the presence or absence of recombinant β-TrCP protein (Creative BioMart, BTRC-2545M).. Then the supernatant was removed, and the beads were thoroughly washed and boiled in 1× loading buffer, followed by Western blot analysis with the indicated antibodies.Then the supernatant was removed, and the beads were thoroughly washed and boiled in 1× loading buffer, followed by Western blot analysis with the indicated antibodies.
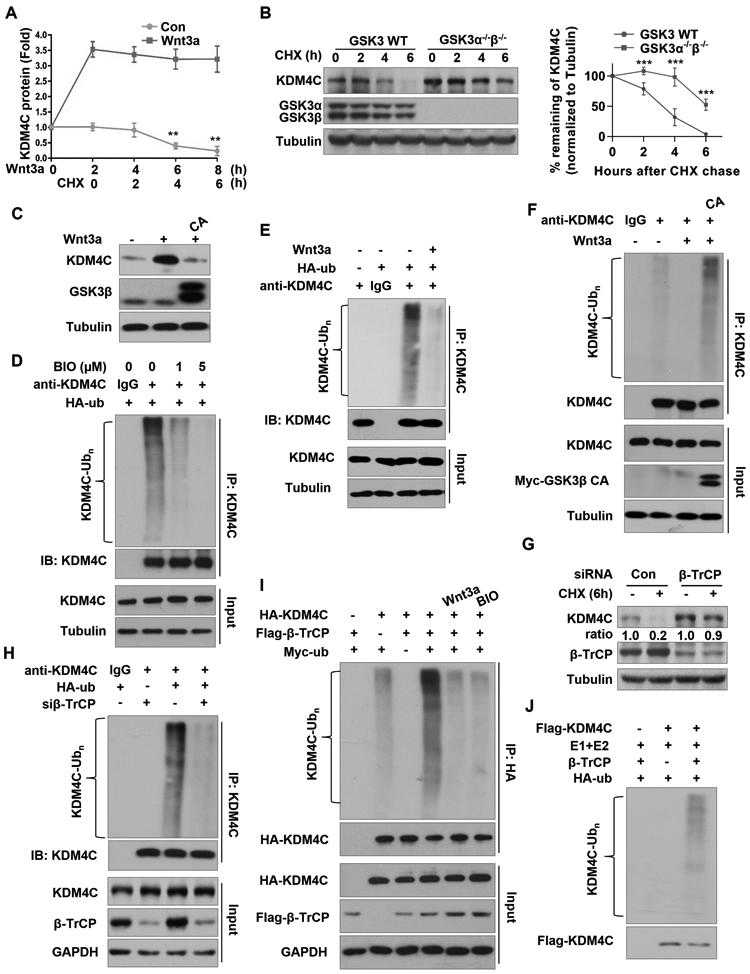
(A) Western blot analysis of KDM4C protein levels in HEK293T cells pretreated with or without 50 ng/ml Wnt3a for 2 hours, and then treated with 100 ng/ml CHX for indicated hours. Band intensities of KDM4C from three independent experiments were quantified by scanning densitometry, using the tubulin signal for normalization; results are expressed as KDM4C protein fold change relative to the control group at 0 h. Data are mean ± SD of three independent quantifications, **P < 0.01. (B) Western blot analysis of KDM4C protein levels in wild-type or double-knockout GSK3α/β mouse stem cells treated with 100 ng/ml CHX for 0, 2, 4, or 6 hours. Quantification of band intensities of KDM4C from three independent experiments was done as described in (A). Data are means ± SD of three independent quantifications, ***P < 0.001. (C) Western blot analysis of KDM4C protein levels in HEK293T cells transfected with or without GSK3β CA and treated with or without Wnt3a. (D) BIO inhibits ubiquitination of KDM4C in vivo. HEK293T cells transfected with HA-ubiquitin (HA-ub), were treated with or without BIO and 25μM MG132 for 4 hours. IP was performed with KDM4C antibody, followed by Western blotting with the indicated antibodies. (E) IP was performed using KDM4C antibody in HEK293T cells transfected with vector or HA-ub plasmids, and treated with MG132 and/or Wnt3a. (F) IP was performed using KDM4C antibody in HEK293T cells transfected with control or HA-ubiquitin and/or GSK3β CA plasmids and treated with MG132 and Wnt3a. (G)
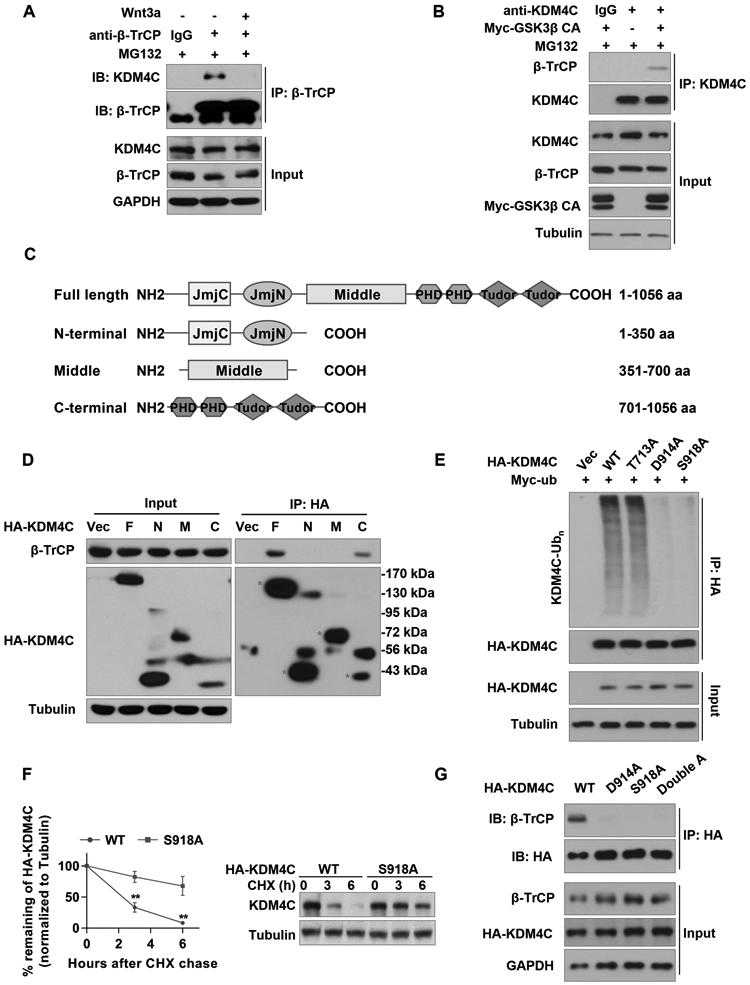
(A) SW1783 cells were treated with or without 50 ng/ml Wnt3a in the presence of MG132. IP was performed using
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All BTRC Products
Required fields are marked with *
My Review for All BTRC Products
Required fields are marked with *



