Monocyte Marker CD Antigen
Related Symbol Search List
- ALCAM
- CD74
- CD84
- CD45
- L-selectin
- CD14
- CD16a
- FCGR3B
- FCGR4
- TLR1
- TLR2
- CD200R
- CD244
- ICAM-2
- Anpep
- Selplg
- CD68
- CSF1R
- CSF2RA
- L1CAM
- CD40
- CD64
- IL1R2
- PVR
- CD226
- IL10RB
- IL13RA1
- IL7R
- LAIR2
- Pvrl2
Immunology Background
Available Resources for Monocyte Marker CD Antigen Research
Creative BioMart is dedicated to providing researchers, scientists, and companies around the world with resources and customized services to help them better understand the biological characteristics of immune cells and promote scientific research and applications in related fields.
Our product portfolio includes recombinant proteins, GMP proteins, protein pre-coupled magnetic beads, cell and tissue lysates, antibodies, chromatography reagents, assay kits, and others targeting monocyte marker CD antigens. |
Our team provides customized services for monocyte marker CD antigens. Whether you need customized tool design or other experimental support services, our team will wholeheartedly provide you with the best service to help you achieve breakthrough research results. |
Our platform provides monocyte marker CD antigens-related information including protein function, interacting proteins, related signal pathways, and other relevant topics, which provide valuable reference and help to researchers. |
About Monocyte Marker CD Antigen
Monocytes are a type of white blood cell that play a critical role in the immune response by engulfing and digesting pathogens, dead cells, and other debris. Monocytes express a variety of cell surface markers, known as CD antigens, which help to identify and characterize these cells in the body. These CD antigens play important roles in immune function, cell signaling, and cell adhesion. Here is an introduction to some commonly used monocyte marker CD antigens:
- CD14 is one of the most well-known monocyte markers. It is a glycosylphosphatidylinositol-anchored protein expressed on the surface of monocytes and macrophages. CD14 acts as a pattern recognition receptor for lipopolysaccharide (LPS), a component of bacterial cell walls. It plays a role in the innate immune response by initiating cellular activation and pro-inflammatory cytokine production.
- CD16, also known as FcγRIII, is a low-affinity Fc receptor expressed on monocytes as well as natural killer (NK) cells and some subsets of neutrophils. CD16 is involved in antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis. Its expression on monocytes is often used to distinguish between classical (CD14++CD16-) and non-classical (CD14+CD16++) monocyte subsets.
- CD11b is a subunit of the integrin receptor known as complement receptor 3 (CR3) or Mac-1. It is expressed on monocytes, macrophages, and other myeloid cells. CD11b is involved in cell adhesion, migration, and phagocytosis. Its expression is upregulated during monocyte activation and recruitment to sites of inflammation.
- CD64, also known as FcγRI, is a high-affinity Fc receptor expressed on monocytes, macrophages, and dendritic cells. CD64 binds to the Fc portion of immunoglobulin G (IgG) antibodies and is involved in phagocytosis, ADCC, and antigen presentation. CD64 expression can be upregulated in response to inflammation or infection.
- CD163 is a scavenger receptor primarily expressed on monocytes and macrophages. It is involved in the clearance of hemoglobin-haptoglobin complexes, contributing to iron homeostasis and anti-inflammatory responses. CD163 is often used as a marker for alternatively activated or anti-inflammatory monocytes/macrophages.
These are just a few examples of monocyte marker CD antigens. There are many other markers that can be used to further characterize and distinguish monocyte subsets based on their phenotype and functional properties. The expression of CD antigens on monocytes can vary depending on various factors, including differentiation stage, tissue microenvironment, and activation state.
The Significance of Monocyte CD Antiges in Disease Diagnosis, Prognosis and Therapeutic Targeting
Monocyte CD antigens have significant implications in disease diagnosis, prognosis, and therapeutic targeting. The expression patterns and alterations of these CD antigens can serve as valuable biomarkers for various diseases. Additionally, targeting specific monocyte CD antigens can be an effective therapeutic strategy in certain conditions. Here's an exploration of the significance of monocyte CD antigens in disease diagnosis, prognosis, and therapeutic targeting:
Disease Diagnosis
- Leukemia and Lymphoma: The expression profile of CD antigens on monocytes can aid in the diagnosis and classification of leukemias and lymphomas. For example, the presence of CD14 and CD64 on monocytes can help distinguish acute monocytic leukemia from other subtypes of acute myeloid leukemia (AML).
- Infectious Diseases: Changes in the expression of CD antigens on monocytes can be indicative of infectious diseases. For instance, altered expression of CD14 and CD16 is observed in sepsis and bacterial infections. Monitoring these changes can assist in diagnosing and monitoring the progression of such infections.
Disease Prognosis
- Cancer: The expression levels of certain CD antigens on monocytes can provide prognostic information in cancer. High expression of CD163 on tumor-associated macrophages (TAMs) within the tumor microenvironment is associated with poor prognosis in various cancers, including ovarian, breast, and colorectal cancer.
- Cardiovascular Disease: The presence of activated monocytes expressing CD40 and CD36 is associated with an increased risk of cardiovascular events. Monitoring the expression of these CD antigens on monocytes can help predict the progression and prognosis of cardiovascular diseases.
Therapeutic Targeting
- Immunotherapy: Monocytes can be targeted for therapeutic interventions in immunotherapy approaches. For example, monoclonal antibodies targeting CD64 or CD163 on monocytes and macrophages have been investigated as potential strategies in the treatment of inflammatory and autoimmune diseases, as well as certain malignancies.
- Anti-inflammatory Strategies: Modulating the function of monocytes and their CD antigens can be a therapeutic approach in inflammatory conditions. Targeting CD14 and CD16 on monocytes has been explored to regulate excessive inflammatory responses in sepsis and autoimmune diseases.
Drug Delivery and Targeting
- Nanomedicine: Monocytes, due to their ability to migrate to sites of inflammation and tumors, can be exploited as carriers for targeted drug delivery. Functionalizing nanoparticles with specific antibodies against monocyte CD antigens allows for specific targeting and delivery of therapeutic agents to disease sites.
Monitoring the expression of these markers provides valuable information for diagnosing diseases, predicting outcomes, and developing targeted therapeutic strategies, ultimately improving patient management and treatment outcomes.
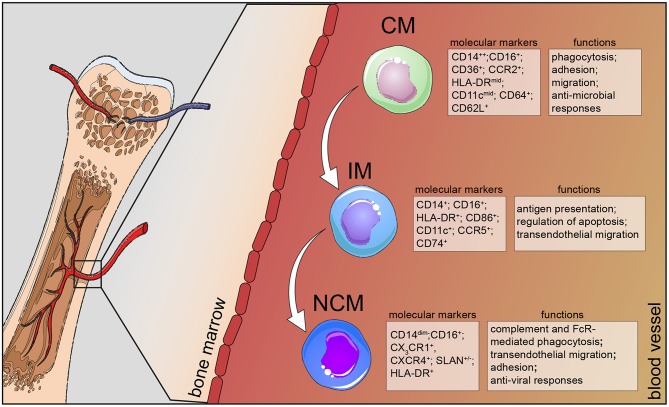 Fig.1 Classical monocytes in humans can be distinguished from the other two subsets by additional markers, such as CD36, CCR2, and CD64 and take part in the host's anti-microbial responses, such as adhesion to the endothelium, migration, and phagocytosis. CM, classical monocytes; IM, intermediate monocytes; NCM, non-classical monocytes. (Wei Q, et al., 2023)
Fig.1 Classical monocytes in humans can be distinguished from the other two subsets by additional markers, such as CD36, CCR2, and CD64 and take part in the host's anti-microbial responses, such as adhesion to the endothelium, migration, and phagocytosis. CM, classical monocytes; IM, intermediate monocytes; NCM, non-classical monocytes. (Wei Q, et al., 2023)
Significance of Monocyte CD Antigens in Diseases
Monocyte CD antigens play a significant role in various diseases, contributing to disease pathogenesis, immune dysregulation, and clinical manifestations. The expression and function of these CD antigens on monocytes can be altered in different disease states, and their significance varies depending on the specific condition. Here are some examples of the significance of monocyte CD antigens in diseases:
Inflammatory Diseases
- Rheumatoid Arthritis (RA): Monocytes in RA patients show increased expression of CD64, CD163, and other activation markers. These alterations contribute to the pathogenesis of RA by promoting pro-inflammatory cytokine production, osteoclast differentiation, and tissue destruction.
- Inflammatory Bowel Disease (IBD): Monocytes in IBD patients exhibit increased expression of CD14, CD16, and CD64. These activated monocytes contribute to intestinal inflammation by producing inflammatory cytokines and promoting tissue damage.
Infectious Diseases
- Sepsis: CD14 is upregulated during sepsis. It plays a critical role in the recognition of bacterial lipopolysaccharide (LPS) and the initiation of the inflammatory response. Dysregulated expression of CD14 on monocytes can contribute to excessive inflammation and organ dysfunction in sepsis.
- HIV/AIDS: Monocytes serve as reservoirs for HIV and can contribute to viral dissemination. CD4, a critical receptor for HIV entry, is expressed on monocytes, allowing viral infection and replication. The interaction between HIV and monocyte CD antigens contributes to the pathogenesis and progression of HIV/AIDS.
Cancer
- Tumor-Associated Macrophages (TAMs): Monocytes can differentiate into TAMs within the tumor microenvironment. TAMs play complex roles in cancer progression, and their phenotype and function are influenced by various CD antigens. For example, high expression of CD163 on TAMs is associated with poor prognosis in several cancers.
- Metastasis: Monocytes can facilitate tumor metastasis through their interaction with cancer cells. CD44, a cell adhesion molecule, is expressed on monocytes and cancer cells, promoting their interaction and facilitating tumor cell extravasation and metastasis.
Cardiovascular Diseases
- Atherosclerosis: Monocytes play a crucial role in the development of atherosclerotic plaques. CD11b and CD36 expression on monocytes is associated with increased adhesion, migration, and uptake of oxidized low-density lipoprotein (LDL), leading to foam cell formation and plaque development.
- Myocardial Infarction (Heart Attack): Elevated levels of CD14+ monocytes and increased expression of CD11b and CD40 on monocytes are associated with an increased risk of myocardial infarction. These activated monocytes contribute to the inflammatory response and tissue damage in the heart.
The significance of monocyte CD antigens in diseases lies in their contribution to disease pathogenesis, immune dysregulation, and clinical outcomes. Understanding the alterations in CD antigen expression and function on monocytes in different diseases can provide insights into disease mechanisms, aid in diagnosis and prognosis, and offer potential targets for therapeutic interventions.
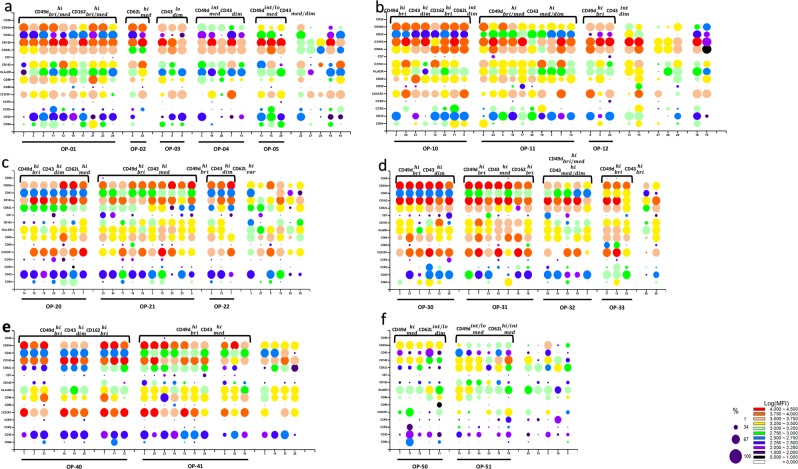 Fig.2 Phenotypes of monocyte subpopulations as analysed. Stained with antibodies directed at various cell surface receptors such as Ig FcR (CD64 and CD32 in addition to CD16), chemokine receptors (CCR2, CCR5, and CX3CR1), antigen presentation and co-stimulatory molecules (HLA-DR, CD86, and CD80), adhesion molecules (CD62L, CD162, CD43, CD49d, and CD56). The expressions of scavenger receptor CD16319,20 and immunoglobulin superfamily molecule CD721 were also determined. (Merah-Mourah F, et al., 2020)
Fig.2 Phenotypes of monocyte subpopulations as analysed. Stained with antibodies directed at various cell surface receptors such as Ig FcR (CD64 and CD32 in addition to CD16), chemokine receptors (CCR2, CCR5, and CX3CR1), antigen presentation and co-stimulatory molecules (HLA-DR, CD86, and CD80), adhesion molecules (CD62L, CD162, CD43, CD49d, and CD56). The expressions of scavenger receptor CD16319,20 and immunoglobulin superfamily molecule CD721 were also determined. (Merah-Mourah F, et al., 2020)
Creative BioMart is committed to providing high-quality products and services to researchers, scientists, and companies around the world to support their research projects and experiments and help them achieve greater success. If you have any questions, requests, or cooperation intentions, please feel free to contact us.
Related References
- Coillard A, Segura E. In vivo Differentiation of Human Monocytes. Front Immunol. 2019;10:1907.
- Kapellos TS, Bonaguro L, Gemünd I, et al. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol. 2019;10:2035.
- Schütt C. Monozytenmarker [Monocyte markers]. Acta Histochem Suppl. 1990;39:333-337.
- Merah-Mourah F, Cohen SO, Charron D, Mooney N, Haziot A. Identification of Novel Human Monocyte Subsets and Evidence for Phenotypic Groups Defined by Interindividual Variations of Expression of Adhesion Molecules. Sci Rep. 2020;10(1):4397.

