Definition and Function of Matrix Metalloproteinases (MMPs)
Matrix metalloproteinases (MMPs) are one of the major families of proteinases that play a key role in the response of cells to the microenvironment. MMPs are a family of calcium-dependent endopeptidases that form a subset of the metzincins. These proteins are characterized by a conserved motif of three histidine residues that bind a zinc ion at the catalytic site. There are approximately 24 human MMPs, and homologues have been identified in other species, including mice, birds, zebrafish, fruit flies, nematodes, plants, and even algae.
Table 1. List of 24 MMPs classified into 6 sub-families according to their substrate specificity (collagenases, gelatinases, stromelysins, matrilysins, mmbrane-bound/associated MMPs, and other MMPs). For each MMP, the gene name, chromo- somic localization, protein name, uniprot database access number, theoretical molecular weight (M W) and isoelectric point (pI) are reported. (Buttacavoli et al., 2021)
MMPs have the combined ability to degrade all components of the ECM. The ECM is a complex and dynamic structure that supports cell adhesion and transmits signals through cell surface adhesion receptors. The ECM is composed of collagens, non-collagenous glycoproteins, and proteoglycans. Alternative components of the ECM, such as tenascin, fibronectin and laminins, are found in tumors and may stimulate cancer progression. The basement membrane (BM) is a specialized ECM that separates epithelial cells from the underlying stroma and represents the first barrier to cancer invasion. It is worth noting that in addition to MMPs, other proteases are capable of degrading ECM substrates. These include the cysteine protease family, which includes cathepsins, and the serine protease family, of which uPA is an important member with recognized functions in ECM degradation and remodeling.
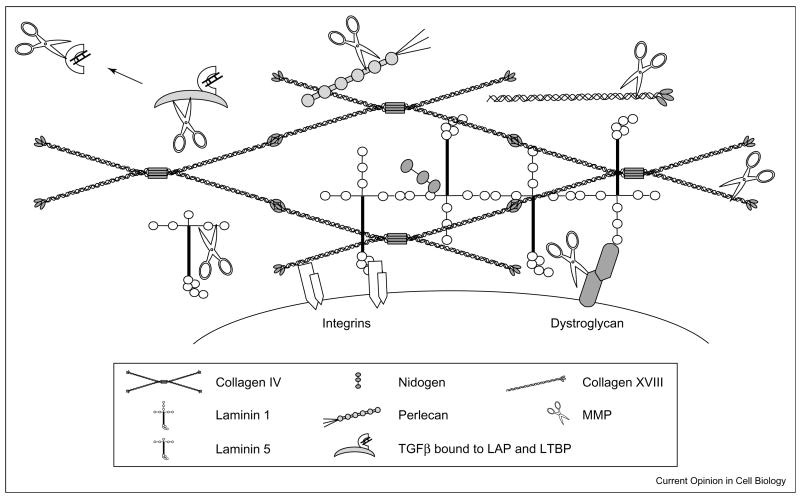
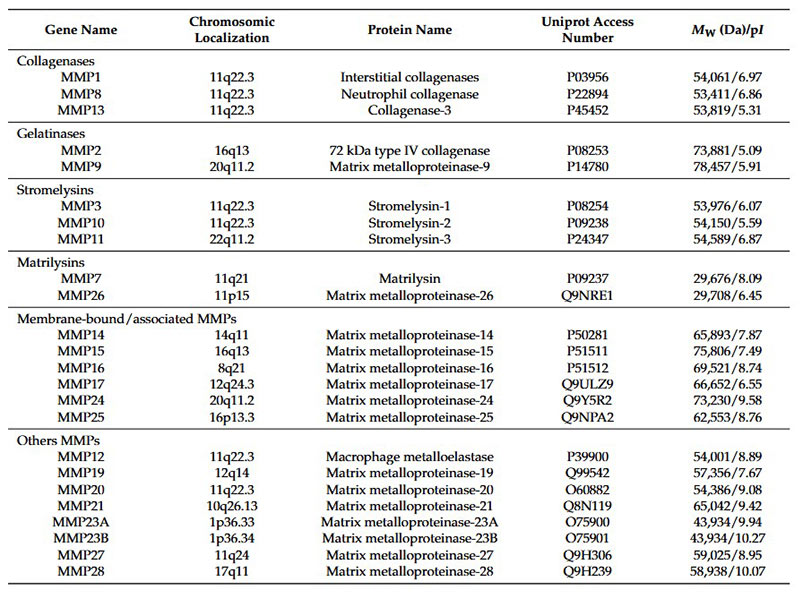
Figure 1. A schematic diagram of some of the proteins that comprise the ECM and the potential for MMP cleavage. The ECM is composed of both laminin and collagen networks. Within ECM network many laminins and collagens are present as well as proteins such as nidogen, perlecan (i.e. heparan sulfate proteoglycans) and fibronectin (not shown). Cell surface receptors such as integrins and dystroglycan interact with proteins of the basement membrane network. Cleavage of ECM molecules releases bioactive fragments that have been referred to as matricryptins or matrikines. Furthermore, growth factors, such as latent TGFβ, are embedded with in ECM, and proteolysis of binding proteins that keep the growth factors in a latent state is a major activation pathway. In addition, some of the cell surface receptors, such as dystroglycan, are targets for proteolysis. Cleavage of these types of molecules breaks cell–ECM contact. (Mott and Werb, 2004)
In addition to degradation of ECM components and activation of other MMPs, MMP activity is responsible for the availability of active growth factors and cytokines such as insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF), FGF receptor 1 (FGFR1), transforming growth factor-β (TGF-β), heparin-binding EGF (HB-EGF), tumor necrosis factor-α (TNF-α), and IL-8. For example, MMPs can cleave the latent forms of TGF-β, converting them into their active forms, which then interact with their receptors to initiate signaling pathways. Additionally, MMPs facilitate the release of ectodomains of growth factors, further regulating their bioavailability and activity. Through these mechanisms, MMPs significantly influence the extracellular environment, affecting the activation and availability of growth factors and cytokines, which in turn regulate various physiological and pathological processes.
Structural Diversity of MMPs
The MMPs were initially classified based on their specificity for ECM components: collagenases, gelatinases, stromelysins and matrilysins. As the list of MMPs grew, a numbering system has been adapted, and the MMPs are now classified according to their structure. There are seven distinct structural classes of MMPs: four are secreted and three are membrane-type MMPs (MT-MMPs).
All MMPs share a basic structural organization consisting of a signal peptide domain that targets them for secretion, a pro-peptide domain necessary to maintain enzyme latency, and an N-terminal catalytic domain. Most MMPs also have a hinge region and a C-terminal hemopexin-like domain that contribute to substrate specificity and interactions with endogenous inhibitors. The MT-MMPs localize to the plasma membrane through a C-terminal transmembrane domain (MT1-, MT2-, MT3-, and MT5-MMP) or through a glycosylphosphatidylinositol anchor (MT4- and MT6-MMP), The MT-MMPs also have an additional insertion of basic residues between the pro-peptide and the catalytic domain, This furin-like cleavage site is also present in three secreted MMPs (MMP-11, MMP-21, and MMP-28). MMP-23 is an exceptional membrane-bound MMP. It has unique cysteine-rich, proline-rich and IL-1 receptor type II-like domains and may be initially anchored by an N-terminal transmembrane domain prior to propeptide processing.
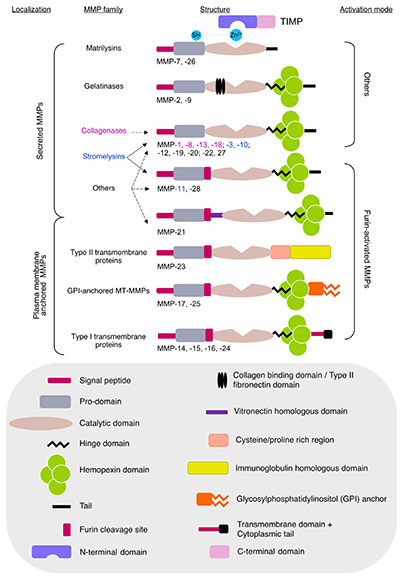
Figure 2. Schematic representation of MMPs and TIMPs’ structural domains. MMPs are classified based on their sequence homology but also on their cellular localization. For instance, matrilysins, gelatinases, stromelysins, and collagenases are secreted MMPs whereas membrane type MMPs (MT-MMPs) and GPI-anchored proteinases are bound to the cell membrane. MMPs can be classified based on their ability to be activated by furin. The different domains of MMPs and TIMPs are indicated in the gray box. (Molière et al., 2023)
Physiological Roles of MMPs
The evolution of the matrix metalloproteinase (MMP) family has resulted in considerable structural diversity, reflecting the complex biological processes in which these enzymes play a key role. MMPs are involved in a wide range of physiological and pathological functions, from tissue remodeling to reproductive processes.
MMPs in Reproductive Processes
MMPs are highly expressed during various reproductive processes, including the menstrual cycle, ovulation, and involution of reproductive organs such as the uterus, breast, and prostate. In particular, MMP-7, MMP-3, MMP-10, MMP-11, and MMP-2 are produced during the active phases of the estrous cycle in mice, whereas MMP-8 and MMP-13 are upregulated during postpartum uterine involution. Interestingly, mutant mice deficient in specific MMPs do not show significant reproductive dysfunction, suggesting that functional redundancy among MMPs may compensate for the loss of individual enzymes.
Figure 3. Global schematic view of matrix metalloproteinases (MMPs) and protein expression profile of their modulators during hormone-induced estrous cycle in prepubescent rats. Note the apparent inverse expression pattern of MMPs inhibitor Reck compared to MMPs and its inducer Sparc. (Levin et al., 2018)
MMP-9's Role in Early Development
MMP-9, produced by invading trophoblasts, plays a critical role in early implantation during pregnancy. Studies with MMP-9-deficient mice have highlighted the involvement of the enzyme in various developmental processes. These mice have defects in endochondral bone formation, delayed apoptosis of hypertrophic chondrocytes at skeletal growth plates, and defective vascularization.
MMPs in Tissue Remodeling
MMPs are critical for tissue remodeling, a process involved in many biological activities such as wound healing, angiogenesis and tissue differentiation. For example, MMP-2 and MMP-3 regulate the branching morphogenesis of the mammary gland during puberty. In wound healing, MMP-1 proteolytically activates keratinocytes and facilitates their migration to re-epithelialize the wound surface. MMP-3-deficient mice show impaired wound contraction, further highlighting the critical role of MMPs in tissue remodeling.
MMPs in Angiogenesis
Matrix metalloproteinases are also required for angiogenesis, the formation of new blood vessels. Endothelial cells express a number of MMPs that are essential for their proliferation, migration and tube formation during angiogenesis. MMP expression and activity in endothelial cells can be modulated by angiogenic factors. Increased MMP expression has been observed in blood vessels under both physiological and pathological conditions. For example, MMP-1 is upregulated in microvascular endothelial cells during embryonic angiogenesis, whereas MMP-9 and MMP-19 are increased in endothelial cells in rheumatoid arthritis synovium.
Despite the critical role of MMPs in angiogenesis, most MMP knockout mice do not exhibit overt angiogenic defects, likely due to overlapping and compensatory functions of other MMPs. MMP-9 knockout mice show abnormal skeletal growth plate angiogenesis, but this is compensated over time. MMP-2 deficient mice show reduced vascularization in response to certain challenges. However, double knockout of MMP-2 and MMP-9 leads to severely impaired choroidal neovascularization, highlighting the synergistic effect of these proteases in angiogenesis.
MMPs regulate angiogenesis not only by promoting extracellular matrix degradation but also by acting as pericellular fibrinolysins during neovascularization. Many MMP family members exhibit a dual ability to either mobilize or activate pro-angiogenic factors or angiogenic inhibitors, further contributing to their complex role in vascular remodeling and angiogenesis.
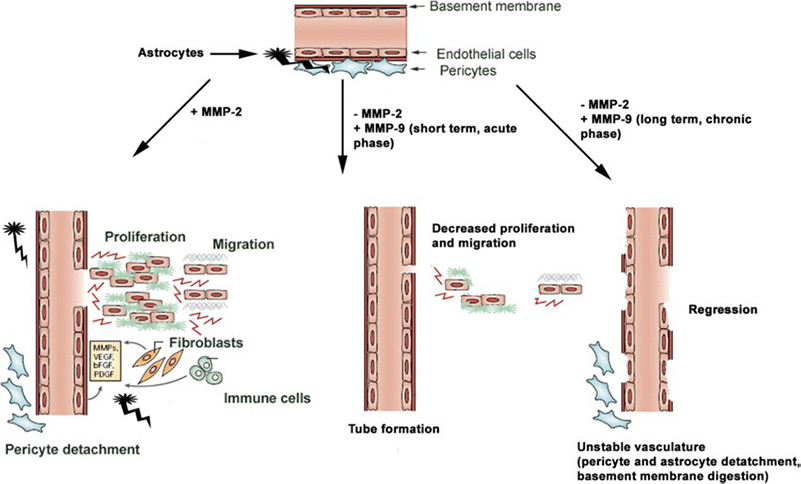
Figure 4. Role of MMP-2 and MMP-9 on angiogenesis is time and context dependent. MMP-2 modulates endothelial proliferation and tube formation, whereas MMP-9 transiently supports only the latter. However, prolonged exposure to MMP-9 leads to pericyte detachment and vascular regression. (Trivedi et al., 2019)
References
- Buttacavoli M, Di Cara G, Roz E, Pucci-Minafra I, Feo S, Cancemi P. Integrated multi-omics investigations of metalloproteinases in colon cancer: focus on MMP2 and MMP9. IJMS. 2021;22(22):12389. doi:10.3390/ijms222212389.
- Curry TE, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428-465. doi:10.1210/er.2002-0005
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. Journal of Cell Science. 2008;121(3):255-264. doi:10.1242/jcs.006064
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161-174. doi:10.1038/nrc745
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3(1):27-45. doi:10.1093/molehr/3.1.27
- Levin G, Coelho TM, Nóbrega NG, Trombetta-Lima M, Sogayar MC, Carreira ACO. Spatio-temporal expression profile of matrix metalloproteinase (MMP) modulators Reck and Sparc during the rat ovarian dynamics. Reproductive Biology and Endocrinology. 2018;16(1):116. doi:10.1186/s12958-018-0422-2
- Molière S, Jaulin A, Tomasetto CL, Dali-Youcef N. Roles of matrix metalloproteinases and their natural inhibitors in metabolism: insights into health and disease. IJMS. 2023;24(13):10649. doi:10.3390/ijms241310649
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Current Opinion in Cell Biology. 2004;16(5):558-564. doi:10.1016/j.ceb.2004.07.010
- Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14(5):624-632. doi:10.1016/s0955-0674(02)00363-0
- Trivedi A, Noble-Haeusslein LJ, Levine JM, Santucci AD, Reeves TM, Phillips LL. Matrix metalloproteinase signals following neurotrauma are right on cue. Cell Mol Life Sci. 2019;76(16):3141-3156. doi:10.1007/s00018-019-03176-4
- Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP). In: Current Topics in Developmental Biology. Vol 54. Elsevier; 2003:1-74. doi:10.1016/S0070-2153(03)54004-2
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

