Recombinant Full Length Human hexokinase 2 Protein, His tagged
| Cat.No. : | HK2-41H |
| Product Overview : | Recombinant human Hexokinase 2 (Full Length), fused to His tag at N-terminus, was expressed in E. coli and purified by using conventional chromatography techniques. |
| Availability | January 27, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Human |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | 1-917aa |
| Description : | Hexokinases phosphorylate glucose to produce glucose-6-phosphate, the first step in most glucose metabolism pathways. This gene encodes hexokinase 2, the predominant form found in skeletal muscle. It localizes to the outer membrane of mitochondria. Expression of this gene is insulin-responsive, and studies in rat suggest that it is involved in the increased rate of glycolysis seen in rapidly growing cancer cells. |
| Tag : | N-His |
| Form : | Liquid |
| Bio-activity : | > 12,000 pmol/min/μg. One unit will convert 1pmole of D-Glucose to D-Glucose-6-phosphate per minute at pH8.0 at 37 centigrade. |
| Molecular Mass : | 104.1 kDa (937aa) |
| AA Sequence : | |
| Purity : | > 85% by SDS-PAGE |
| Applications : | SDS-PAGE, Enzyme Activity |
| Storage : | Can be stored at +2 to +8 centigrade for 1 week. For long term storage, aliquot and store at -20 to -80 centigrade. Avoid repeated freezing and thawing cycles. |
| Concentration : | 1 mg/mL (determined by Bradford assay) |
| Storage Buffer : | 20mM Tris-HCl buffer (pH 8.0) containing 10% glycerol |
| Notes : | For research use only. This product is not intended or approved for human, diagnostics or veterinary use. |
| References : | 1. Jon E. et al.,(2003) J.Exp Biology. 206 : 2049-2057. 2. Furuta H. et al.,(1996) Genomics. 36(1):206-9. |
| Publications : |
| Gene Name | HK2 hexokinase 2 [ Homo sapiens (human) ] |
| Official Symbol | HK2 |
| Synonyms | HK2; hexokinase 2; HKII; HXK2; hexokinase-2; hexokinase type II; hexokinase-2, muscle; hexokinase-B; muscle form hexokinase; EC 2.7.1.1 |
| Gene ID | 3099 |
| mRNA Refseq | NM_000189 |
| Protein Refseq | NP_000180 |
| MIM | 601125 |
| UniProt ID | P52789 |
| ◆ Recombinant Proteins | ||
| HK2-2862R | Recombinant Rat HK2 Protein | +Inquiry |
| HK2-359HFL | Recombinant Full Length Human HK2 Protein, C-Flag-tagged | +Inquiry |
| HK2-2352H | Recombinant Human HK2 Protein (Trp619-Arg917), N-His tagged | +Inquiry |
| HK2-12296Z | Recombinant Zebrafish HK2 | +Inquiry |
| HK2-329H | Recombinant Human HK2 Protein, MYC/DDK-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| HK2-5508HCL | Recombinant Human HK2 293 Cell Lysate | +Inquiry |
A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis
Journal: Nature Communications PubMed ID: 35173161 Data: 2022/2/16
Authors: Catherine S. Blaha, Gopalakrishnan Ramakrishnan, Nissim Hay
Article Snippet:Enzyme-linked immunosorbent assays were conducted in Pierce Nickel-8-well-strip-coated plates.Enzyme-linked immunosorbent assays were conducted in Pierce Nickel-8-well-strip-coated plates.. The plates were coated with His-HK2 (50 nM; Creative BioMart) in coating buffer and incubated overnight at 4 °C.. Unbound proteins is then washed and blocking buffer is added for 1 h at room temperature.Unbound proteins is then washed and blocking buffer is added for 1 h at room temperature.
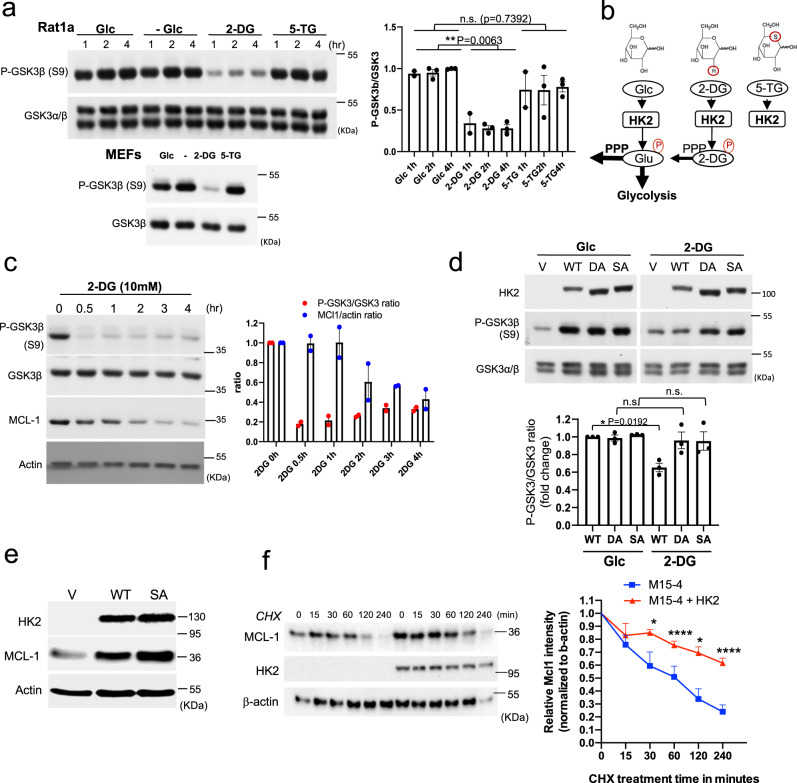
a Rat1a cells or MEFs were incubated in glucose-free medium in the absence (?) or presence of 10 mM glucose (Glc), 2-DG or 5-TG. Immunoblots showing GSK3β phosphorylation, at the indicated time points after incubation. Bar graph shows densitometric quantification of pGSK3β/GSK3α/β ratio from three independent experiments in Rat1a cells ( n = 3). Data are presented as the mean ± SEM. ** p = 0.0063; n.s. p = 0.7392; one-way ANOVA was used to calculate significance. b Schematic depicting the structures of glucose (Glc), 2-DG, and 5-TG and their utilization by HK inside cells. Like Glc, 2-DG can be phosphorylated by
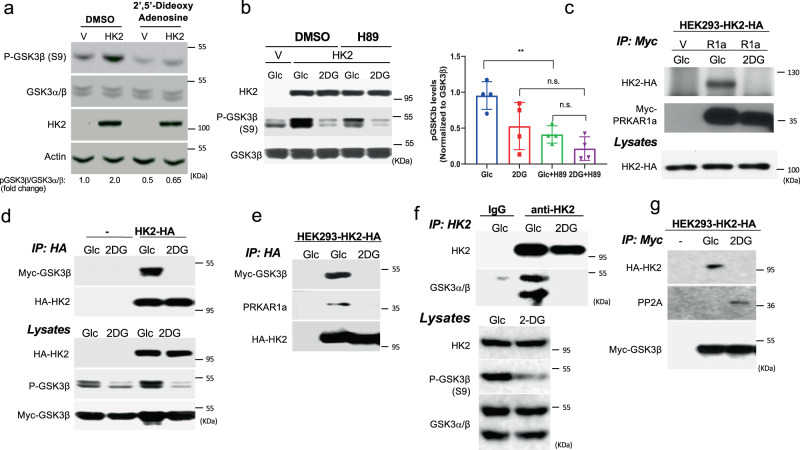
a Immunoblot showing GSK3β phosphorylation following
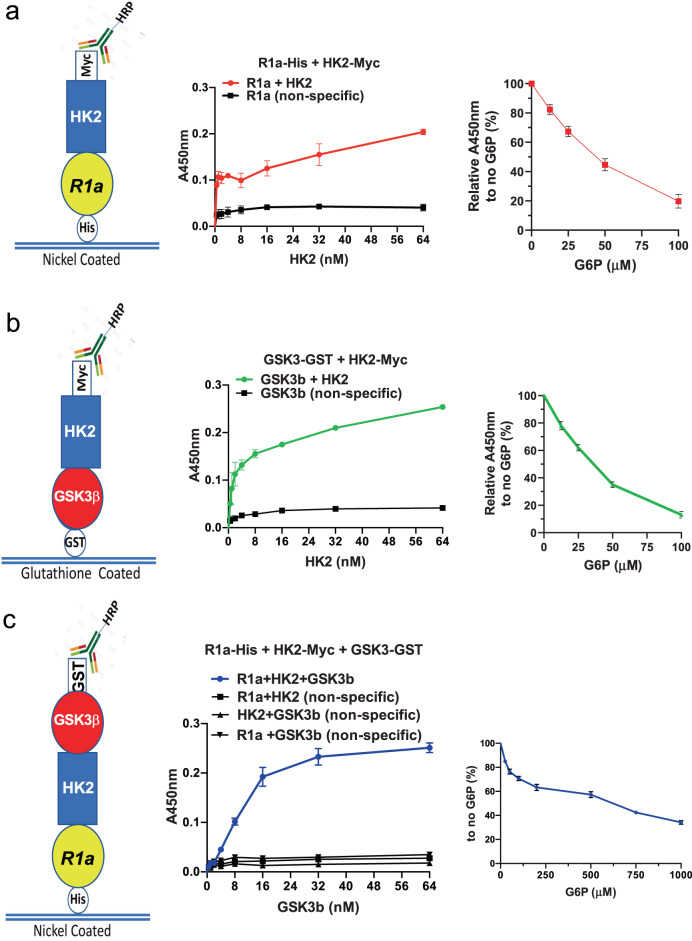
a Nickel-coated 96-well plates were incubated overnight at 4 °C with His-PRKAR1a (50 nM) and then incubated with
A novel non-catalytic scaffolding activity of Hexokinase 2 contributes to EMT and metastasis
Journal: bioRxiv Data: 2021/5/10
Authors: Blaha Catherine, Ramakrishnan Gopalakrishnan, Hay Nissim
Article Snippet:PrePrint: Enzyme-linked immunosorbent assays were conducted in Pierce Nickel- 8-well-strip coated plates.Enzyme-linked immunosorbent assays were conducted in Pierce Nickel- 8-well-strip coated plates.. The plates were coated with His-HK2 (50nM; Creative BioMart) in coating buffer and incubated overnight at 4°C.. Unbound proteins is then washed and blocking buffer is added for 1h at room temperature.Unbound proteins is then washed and blocking buffer is added for 1h at room temperature.
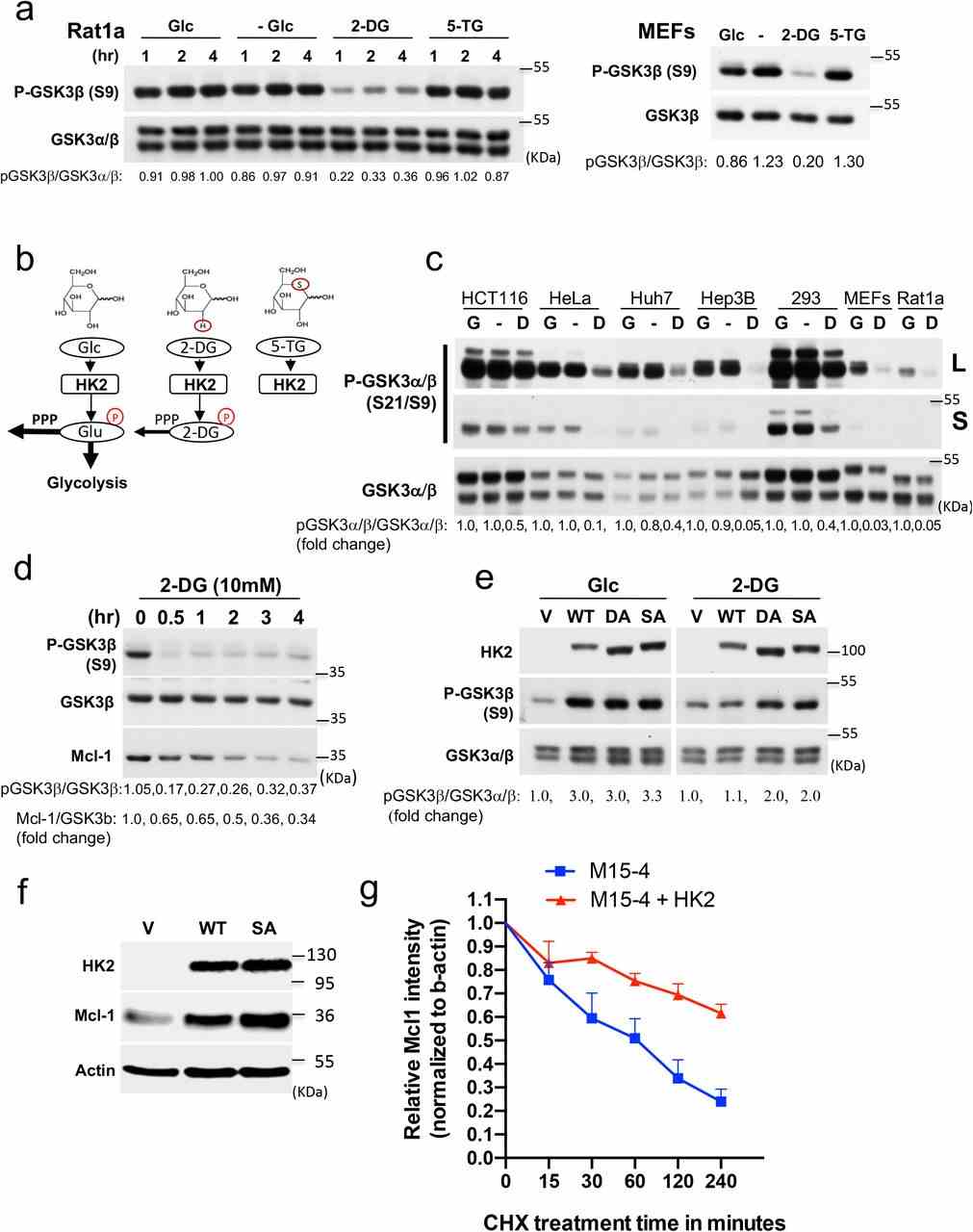
a. Rat1a cells or MEFs were incubated in glucose free medium in the absence (-) or presence of 10mM glucose (Glc), 2-DG or 5-TG. Immunoblots showing GSK3 β phosphorylation, at the indicated time points after incubation. b. Schematic depicting the structures of glucose (Glc), 2-DG, and 5-TG and their utilization by HK inside cells. Similar to Glc, 2-DG can be phosphorylated by
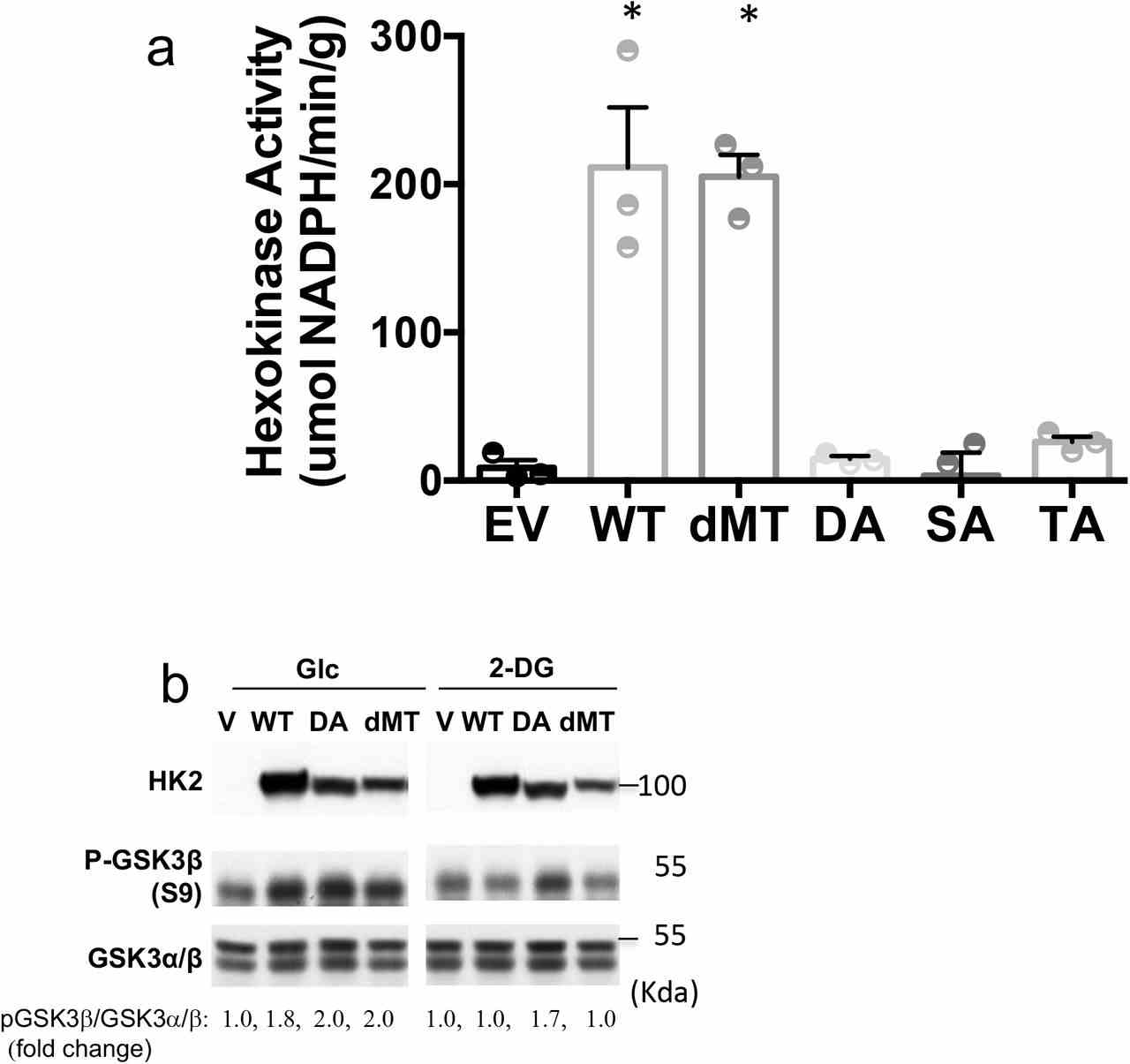
a. Hexokinase activity in M15-4 CHO cells expressing empty vector (EV), WT
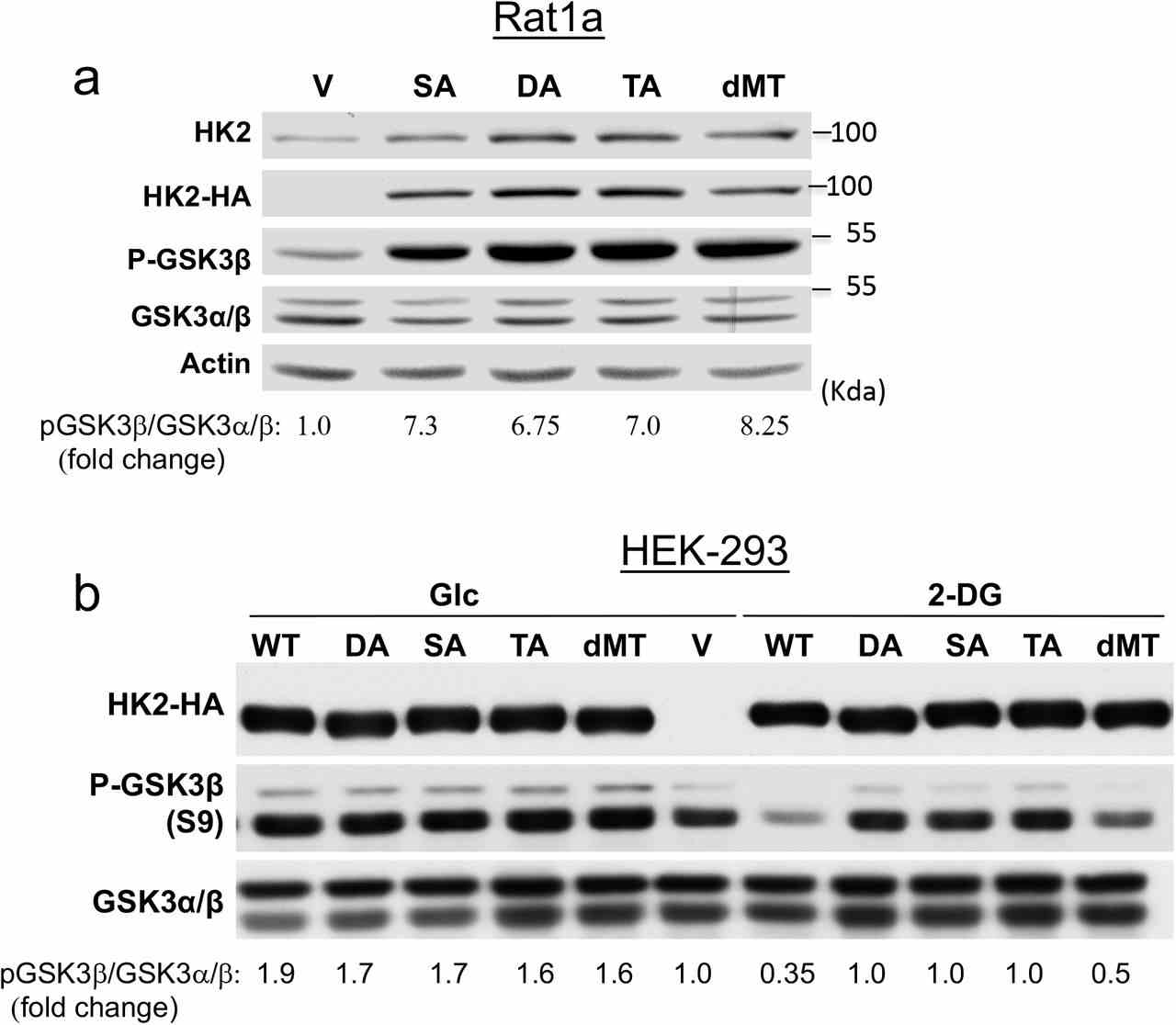
a. The effect of WT
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All HK2 Products
Required fields are marked with *
My Review for All HK2 Products
Required fields are marked with *



