Recombinant Anoxybacillus pyrK Protein (Met1-Leu256), N-His tagged
| Cat.No. : | pyrK-1394A |
| Product Overview : | Recombinant Anoxybacillus pyrK (Met1-Leu256) fused with the N-His tag was expressed in E. coli. |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Anoxybacillus |
| Source : | E.coli |
| Tag : | His |
| Protein Length : | Met1-Leu256 |
| Form : | Lyophilized powder/frozen liquid |
| Molecular Mass : | 29.92 kDa |
| Purity : | >90% as determined by SDS-PAGE. |
| Notes : | For research use only. |
| Storage : | Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8 centigrade for one week. Store at -20 to -80 centigrade for twelve months from the date of receipt. |
| Storage Buffer : | Supplied as solution form in PBS, pH 7.5 or lyophilized from PBS, pH 7.5. |
| Reconstitution : | Reconstitute in sterile water for a stock solution. |
| Shipping : | They are shipped out with dry ice/blue ice unless customers require otherwise. |
| Official Symbol | pyrK |
| Synonyms | pyrK; Dihydroorotate dehydrogenase B (NAD (+)), electron transfer subunit; pyrK; Dihydroorotate oxidase B |
| UniProt ID | A0A160F951 |
| ◆ Recombinant Proteins | ||
| PYRK-0488B | Recombinant Bacillus subtilis PYRK protein, His-tagged | +Inquiry |
| pyrK-1394A | Recombinant Anoxybacillus pyrK Protein (Met1-Leu256), N-His tagged | +Inquiry |
Case 1: Amiri-Dashatan N, et al. Ann Parasitol. 2020
In Iran and many other countries, cutaneous leishmaniasis is prevalent. Since differential gene expression plays an important role in the outcome of infection, researchers compared the relative gene expression values of pyruvate kinase (PyrK) and tryptophan peroxidase (TryP) in the proflagellate phase of L. major and L. tropica isolates from Iran. Clinical isolates of CL patients were collected from an endemic area of leishmaniasis in Iran and identified by PCR-RFLP. Subsequently, they evaluated the expression levels of PyrK and Tryp genes in L. major and L. tropica using real-time PCR. Through this comparison, researchers observed up-regulation of PyrK and Tryp genes in the proflagellate phase. In addition, the mean mRNA expressions of PyrK and Tryp genes in L. major were 1.69 times and 3.72 times higher than those in L. tropica isolates, respectively. The results of this study may provide a new perspective for further exploring the link between parasite gene expression levels and disease pathology. The contribution of species-specific parasite factors to virulence and pathogenicity in the host may be mainly due to the differential regulation of conserved genes between species.
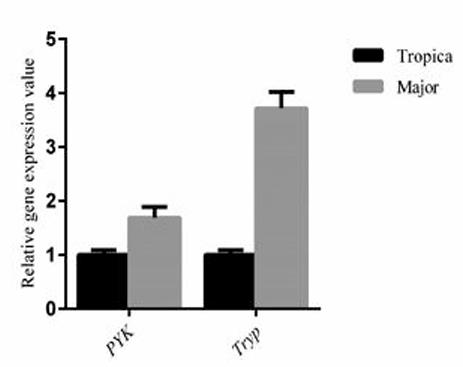
Fig1. Relative gene expression pattern of two correspond genes of differential expressed proteins between L. major and L. tropica metacyclic stage by real-time PCR.
Case 2: Kawasaki S, et al. Appl Environ Microbiol. 2009
Previous studies have shown the existence of a microaerobic Bifidobacterium species that grow better under aerobic conditions than without oxygen in liquid shake culture. The difference in aerobic growth characteristics between O2-sensitive and microaerobic species is due to the existence of H2O2 generation system in the growth medium. In this study, researchers purified and identified NADH oxidase, an enzyme believed to be a key in the production of H2O2. Bifidobacterium bifidum, an O2-sensitive bacterium that is also a model species of Bifidobacterium, detected a major active NADH oxidase component and a minor active NAD(P)H oxidase component in the first column chromatography of its enzyme purification process. After further purification of the main active component, the N-terminal sequence was determined to be a homolog of B-type dihydroorotic dehydrogenase (DHOD), consisting of PyrK (31 kDa) and PyrDb (34 kDa) subunits. The genes encoding PyrK and PyrDb reside in an operon structure. The purified enzyme was found to be a heterotetramer, displaying a spectrum typical of flavin proteins and identifying flavin mononucleotides and flavin adenine dinucleotides as cofactors. The purified enzymes were identified as the enzymes that catalyzed the DHOD reaction and also catalyzed the NADH oxidase reaction to form H2O2 in the presence of O2. Kinetic parameters suggest that this enzyme may be involved in H2O2 production in highly aerated environments.
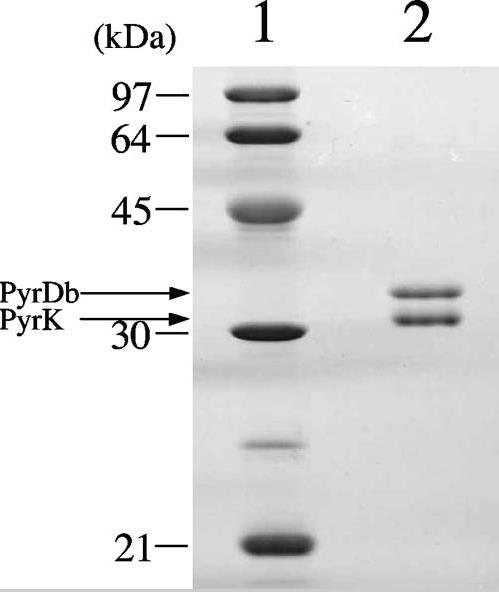
Fig1. SDS-PAGE of purified B. bifidum DHOD.
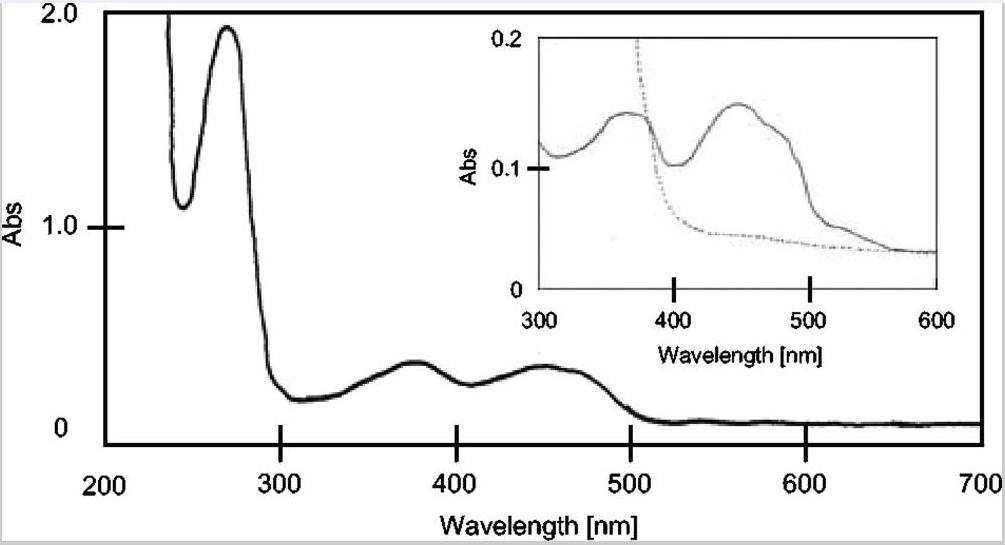
Fig2. Absorption spectrum of DHOD.
Recombinant Anoxybacillus pyrK Protein is a protein found in Bacillus thermophilus. This protein is closely related to the purine biosynthesis pathway, in particular it is involved in the synthesis of pyrimidine nucleotides, which are an important component of cellular DNA and RNA synthesis.
The PyrK protein typically encodes an enzyme that is responsible for catalyzing the synthesis of hypoxanthine nucleotides (IMP), a key step in the purine pathway. In recombinant form, PyrK protein can be used in a variety of biological and medical studies, including but not limited to enzyme kinetics studies, drug screening, and potential therapeutic applications in certain genetic diseases. Because of the role of PyrK protein in purine metabolism, it may be important for the development of inhibitors or activators that target specific metabolic pathways that may be used to treat certain cancers or other diseases associated with abnormal purine metabolism.
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All pyrK Products
Required fields are marked with *
My Review for All pyrK Products
Required fields are marked with *



