Recombinant Mouse Cd19 protein, His-tagged
| Cat.No. : | Cd19-3308M |
| Product Overview : | Recombinant Mouse Cd19 Protein (Met1-Gly287) is produced by HEK293 Cells expression system. This protein is fused with a His tag at the C-terminal. |
| Availability | January 29, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Download
| Species : | Mouse |
| Source : | HEK293 |
| Tag : | His |
| Protein Length : | Met1-Gly287 |
| Form : | Lyophilized from sterile PBS, pH 7.4 |
| Molecular Mass : | The recombinant mouse CD19 consists of 282 amino acids and has a predicted molecular mass of 31.1 kDa. It migrates as approximately 51.8 kDa band in SDS-PAGE under reducing conditions. |
| Endotoxin : | < 1.0 EU per μg protein as determined by the LAL method. |
| Purity : | > 90 % as determined by SDS-PAGE |
| Storage : | Samples are stable for up to twelve months from date of receipt at -20°C to -80°C Store it under sterile conditions at -20°C to -80°C. It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles. |
| Reconstitution : | It is recommended that sterile water be added to the vial to prepare a stock solution of 0.2 ug/ul. Centrifuge the vial at 4°C before opening to recover the entire contents. |
| Gene Name | Cd19 CD19 antigen [ Mus musculus ] |
| Official Symbol | Cd19 |
| Synonyms | CD19; CD19 antigen; B-lymphocyte antigen CD19; differentiation antigen CD19; AW495831; |
| Gene ID | 12478 |
| mRNA Refseq | NM_009844 |
| Protein Refseq | NP_033974 |
| ◆ Recombinant Proteins | ||
| CD19-0732H | Recombinant Human CD19 Protein, GST-Tagged | +Inquiry |
| CD19-3065R | Recombinant Rat CD19 protein, His-tagged | +Inquiry |
| CD19-3309HF | Recombinant Human CD19 Protein, His-tagged, FITC conjugated | +Inquiry |
| CD19-01M | Active Recombinant Mouse CD19 Chimera Protein | +Inquiry |
| CD19-408HF | Recombinant Human CD19 Protein, Fc-tagged, FITC conjugated | +Inquiry |
| ◆ Native Proteins | ||
| CD19-59M | Active Recombinant Mouse CD19 Protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| CD19-2005HCL | Recombinant Human CD19 cell lysate | +Inquiry |
| CD19-2164MCL | Recombinant Mouse CD19 cell lysate | +Inquiry |
Homology-Directed Recombination for Enhanced Engineering of Chimeric Antigen Receptor T Cells
Journal: Molecular Therapy. Methods & Clinical Development PubMed ID: 28345004 Data: 2017/1/10
Authors: Malika Hale, Baeckseung Lee, David J. Rawlings
Article Snippet:For LV transduction, 24 hr post-activation, LV was added to cells at a MOI of 20, followed by overnight incubation, then media was replenished, and cells cultured using standard conditions.For LV transduction, 24 hr post-activation, LV was added to cells at a MOI of 20, followed by overnight incubation, then media was replenished, and cells cultured using standard conditions.. At 5 to 6 hr post-LV or HDR, TCR and CAR expression and T cell phenotype were determined by flow cytometry after labeling with APC anti-CD62L (Dreg-56), AlexaFluor488 anti-CD3 (SK7), PE anti-CD45RA (HI100), all from BioLegend, and recombinant PE-CD19-His (Creative BioMart).
![Equivalent Functional Responses of LV- and HDR-Generated CD19-CAR T Cells to CD19 pos Cell Line Targets In Vitro (A) Flow cytometry showing BFP and surface CAR expression by Protein L staining in untreated (mock) T cells and BFP-enriched CCR5 [BFP], CCR5 [CD19CAR-BFP], and LV CD19CAR-BFP T cells. (B) Specific killing of CD19 pos K562 in a mixed target assay at 48 hr post-mixing with T cells at increasing E:T ratios. The specific lysis capacity of CCR5 [CD19CAR-BFP] or LV CD19CAR-BFP T cells is significant versus CCR5 [BFP] T cells by ANOVA (p < 0.001), but not significantly different from each other by ANOVA, or by unpaired t test at any E:T ratio. (C–E) % CD107a + cells of live CD3 + cells in a degranulation assay using CCR5-edited or LV T cells stimulated with CD19 pos or CD19 neg K562. % CD137 expression on CD3 + cells at 6–8 (D) or 24 (E) hr post-mixing with target cell lines. The significance shown is by unpaired two-tailed t test. The error bars represent SEM. *p < 0.05 and **p < 0.0001. CAR T cells were generated from three donors and each experiment was performed in triplicate.](productimages/extendimages/pmc05363294__gr1.jpg)
Equivalent Functional Responses of LV- and HDR-Generated
![Efficient Generation of CD3 ? CD19CAR + T Cells by Integration of CD19CAR into the TRAC Locus (A) Schematic of the TRAC and the homology template containing a second generation CD19CAR construct under the control of the MND promoter. The annotated TRAC-megaTAL cleavage site is located within the first exon of TRAC . The CD19CAR lentiviral construct has an MND promoter regulating the expression of a second generation CD19-targeting CAR combined with a CD8 hinge/trans-membrane (TM) domain and 41BB/CD3ζ intracellular signaling domains. (B) Analysis of CAR and CD3 expression on cells transduced with LV CD19CAR or gene edited to TRAC [CD19CAR]. The representative FACS plots (left) and summary data from three unique donors (right) are shown. The colored quadrants were used for T cell phenotyping in (C), as indicated by arrows. (C) Phenotypic analysis of CD45RA and CD62L T cell differentiation markers in CAR + T cells 6 days post-HDR or LV transduction, with representative FACS plots (left) and summary data from three unique donors (right) is shown. Analysis in (B) and (C) is representative of three unique experiments and at least two different donors per experiment. The error bars represent SEM.](productimages/extendimages/pmc05363294__gr3.jpg)
Efficient Generation of CD3 ? CD19CAR + T Cells by Integration of CD19CAR into the TRAC Locus (A) Schematic of the TRAC and the homology template containing a second generation CD19CAR construct under the control of the MND promoter. The annotated TRAC-megaTAL cleavage site is located within the first exon of TRAC . The CD19CAR lentiviral construct has an MND promoter regulating the expression of a second generation
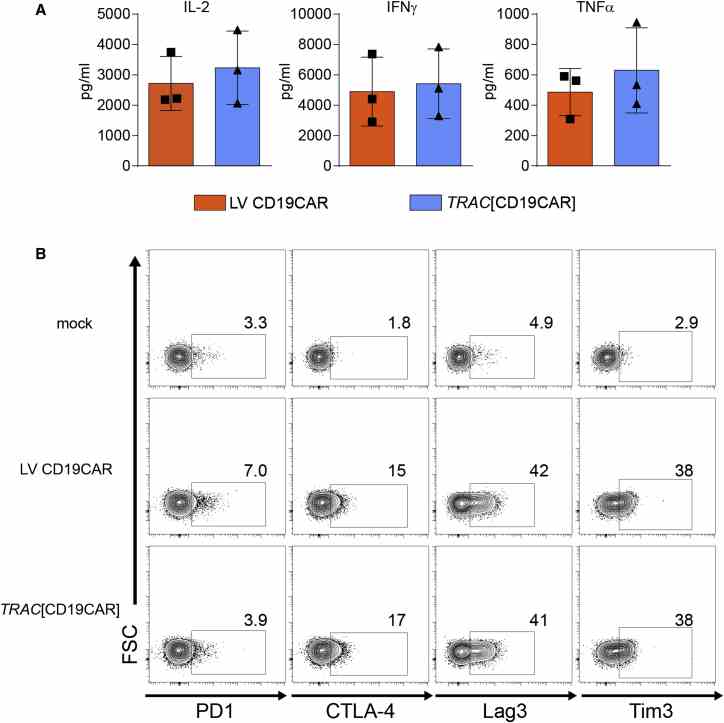
Equivalent In Vitro Function of
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All Cd19 Products
Required fields are marked with *
My Review for All Cd19 Products
Required fields are marked with *



