Osteoclast Markers
Related Symbol Search List
- ACAN
- CD44
- IBSP
- ALCAM
- ALPL
- BGLAP
- BGN
- CALCR
- DLX5
- DMP1
- GABBR1
- IGFBP3
- ITGAV
- MEPE
- Nt5e
- OCSTAMP
- Omd
- OSCAR
- PTH1R
- CD45
- Reg3A
- SCUBE3
- SMOC1
- SMOC2
- SOSTDC1
- SP7
- SPARC
- TBX2
- TBX3
- TBX5
- TCIRG1
- Thy1
- TNFRSF11A
- RANKL
- TPO
- WDR5
Immunology Background
Background
Osteoclasts are specialized cells that play a vital role in bone remodeling and resorption. They are large, multinucleated cells derived from hematopoietic stem cells, specifically from the monocyte/macrophage lineage. Osteoclasts are primarily responsible for breaking down and resorbing bone tissue as part of the bone remodeling process.
Function of Osteoclasts
- Bone Resorption: The primary function of osteoclasts is to resorb bone tissue by secreting enzymes and acids that break down the mineralized matrix of bone. This process releases calcium and other minerals into the bloodstream and helps regulate calcium levels in the body.
- Remodeling Bone: Osteoclasts are essential for the continuous turnover and remodeling of bone tissue. By removing old or damaged bone and creating small cavities, osteoclasts prepare the bone surface for new bone formation by osteoblasts.
- Regulating Bone Density: Osteoclast activity is tightly regulated to maintain the balance between bone resorption and bone formation. Dysregulation of osteoclast function can lead to conditions such as osteoporosis (excessive bone loss) or osteopetrosis (excessive bone density).
Osteoclast markers are specific molecules or proteins that are expressed by osteoclasts or are associated with osteoclast activity. These markers play a crucial role in identifying, characterizing, and studying osteoclasts in the context of bone remodeling, skeletal development, and various bone-related diseases.
Common Osteoclast Markers
Common osteoclast markers used in research and clinical settings for identifying and studying osteoclasts include:
- Tartrate-Resistant Acid Phosphatase (TRAP): TRAP is a specific enzyme highly expressed in osteoclasts and is involved in bone resorption. It is commonly used as a marker to identify and characterize osteoclasts in histological sections or cell cultures.
- Calcitonin Receptor (CTR): CTR is a cell surface receptor expressed on osteoclasts that responds to calcitonin, a hormone that inhibits bone resorption. It is used as a marker to identify osteoclasts and study their regulatory pathways.
- Receptor Activator of Nuclear Factor Kappa-B (RANK): RANK is a receptor expressed on osteoclast precursors that plays a crucial role in osteoclast differentiation and activation in response to RANK Ligand (RANKL). It is a key marker for identifying osteoclast precursor cells.
- Cathepsin K: Cathepsin K is a lysosomal enzyme produced by osteoclasts that is essential for degrading collagen and other proteins in the bone matrix during resorption. It is a specific marker of osteoclast activity.
- Integrin αvβ3 (Vitronectin Receptor): Integrin αvβ3 is a cell adhesion molecule expressed on osteoclasts that is involved in osteoclast attachment to the bone surface and the formation of the sealing zone during bone resorption. It is a key marker for identifying active osteoclasts.
- Carbonic Anhydrase II (CAII): CAII is an enzyme that catalyzes the conversion of carbon dioxide to bicarbonate and protons, contributing to the acidification of the resorption lacuna by osteoclasts. It is used as a marker for osteoclast activity and acid secretion during bone resorption.
Here are other osteoclast markers:
| Classifications | Descriptions |
|---|---|
| Enzymes |
ALPL (Alkaline Phosphatase) is an enzyme involved in the mineralization of bone. It plays a role in osteoblast differentiation and bone formation. Nt5e (Ecto-5'-nucleotidase) is an enzyme that regulates extracellular nucleotide levels and is involved in bone mineralization and osteoclastogenesis. TCIRG1 (T-cell Immune Regulator 1) encodes a subunit of the osteoclast proton pump and is crucial for bone resorption. |
| Cell Surface Receptors and Adhesion Molecules |
ACAN (Aggrecan) is a proteoglycan involved in cell adhesion and signaling processes in the bone microenvironment. CD44 (Cluster of Differentiation 44) is a cell surface glycoprotein involved in cell-cell interactions, adhesion, and migration. ALCAM (Activated Leukocyte Cell Adhesion Molecule) is a cell adhesion molecule that plays a role in cell-cell interactions and migration. CALCR (Calcitonin Receptor) is a receptor for calcitonin, a hormone that regulates bone remodeling by inhibiting osteoclast activity. OCSTAMP (Osteoclast Stimulatory Transmembrane Protein) is a transmembrane protein involved in osteoclast fusion and multinucleation. RANKL (Receptor Activator of Nuclear Factor Kappa-B Ligand) is a cytokine that activates osteoclast differentiation and function through binding to RANK. PTH1R (Parathyroid Hormone 1 Receptor) is a receptor for parathyroid hormone that regulates calcium homeostasis and bone remodeling. Thy1 (Thymocyte Differentiation Antigen 1) is a cell surface glycoprotein involved in cell adhesion and signaling in various cell types. |
| Matrix Proteins and Regulators |
IBSP (Integrin-Binding Sialoprotein) is a matrix protein that binds to integrins and plays a role in cell adhesion and mineralization. BGLAP (Bone Gamma-Carboxyglutamic Acid-Containing Protein), also known as Osteocalcin, is a marker of osteoblast activity and bone formation. DMP1 (Dentin Matrix Protein 1) is a non-collagenous protein involved in mineralization and matrix organization in bone and dentin. MEPE (Matrix Extracellular Phosphoglycoprotein) is a matrix protein involved in phosphate homeostasis and mineralization in bone. SPARC (Secreted Protein, Acidic, and Rich in Cysteine) is a matricellular protein that regulates extracellular matrix assembly and mineralization. |
| Transcription Factors and Regulators |
DLX5 (Distal-Less Homeobox 5) is a transcription factor involved in osteoblast differentiation and skeletal development. SP7 (Osterix) is a transcription factor critical for osteoblast differentiation and bone formation. TBX2, TBX3, TBX5 (T-Box Transcription Factors) are involved in skeletal development and patterning. |
| Other Regulators and Signaling Molecules |
GABBR1 (Gamma-Aminobutyric Acid Type B Receptor Subunit 1) is involved in GABA signaling and may have regulatory functions in bone metabolism. IGFBP3 (Insulin-Like Growth Factor Binding Protein 3) regulates the activity of insulin-like growth factors and may influence bone growth and remodeling. SOSTDC1 (Sclerostin Domain Containing 1) is a secreted protein that may play a role in modulating Wnt signaling in bone and have implications for bone formation and resorption. |
| Other Proteins and Factors |
BGN (Biglycan) is a small leucine-rich proteoglycan that interacts with collagen and may play a role in bone matrix organization. CD45 (Cluster of Differentiation 45) is a protein tyrosine phosphatase expressed on immune cells and may have roles in osteoclast development. SMOC1 (SPARC-Related Modular Calcium-Binding Protein 1) and SMOC2 are members of the SPARC family of proteins and may be involved in extracellular matrix regulation. SCUBE3 (Signal Peptide, CUB, and EGF-Like Domain Containing 3) is a protein containing multiple domains and may have regulatory functions in bone development or homeostasis. OSCAR (Osteoclast-Associated Immunoglobulin-Like Receptor) is a receptor expressed on osteoclasts and may be involved in osteoclast differentiation and activation. WDR5 (WD Repeat Domain 5) is a protein associated with chromatin remodeling and gene expression regulation, which may impact osteoclast differentiation or function. |
The listed osteoclast markers encompass a diverse array of proteins involved in various aspects of bone metabolism, including cell signaling, adhesion, matrix regulation, transcriptional control, and regulatory functions. Understanding the roles of these markers can provide valuable insights into osteoclast biology, bone remodeling processes, and the development of therapeutic strategies for bone-related disorders.
Importance of Osteoclast Markers
Identifying and studying osteoclast markers is of significant importance in both research and clinical settings for the following reasons:
| Importance | Descriptions |
|---|---|
| Understanding Bone Remodeling |
Significance: Osteoclasts play a critical role in bone remodeling by resorbing old or damaged bone tissue, which is followed by new bone formation. Osteoclast markers help researchers and clinicians track the activity of osteoclasts and understand the mechanisms involved in bone resorption and formation. Impact: By studying osteoclast markers, researchers can gain insights into the dynamic process of bone remodeling, which is essential for maintaining bone health and integrity. |
| Disease Diagnosis and Monitoring |
Significance: Abnormal osteoclast activity is associated with various bone disorders such as osteoporosis, Paget's disease, and bone metastases. Osteoclast markers provide valuable diagnostic tools for identifying these conditions and monitoring disease progression. Impact: Monitoring changes in the expression of osteoclast markers in clinical settings can help assess disease severity, response to treatment, and overall bone health in patients with bone-related disorders. |
| Targeted Therapies |
Significance: Osteoclast markers are crucial for developing targeted therapies that modulate osteoclast activity. Understanding the expression and function of these markers allows for the development of drugs that specifically target osteoclasts to treat bone diseases. Impact: Targeted therapies that modulate osteoclast function can help regulate bone resorption, promote bone formation, and improve outcomes for patients with conditions such as osteoporosis and bone metastases. |
| Drug Development and Screening |
Significance: Osteoclast markers serve as valuable targets for drug development and screening. Researchers can use these markers to evaluate the efficacy of potential therapeutic agents in modulating osteoclast activity. Impact: By targeting specific osteoclast markers, researchers can develop novel treatments that selectively inhibit or enhance osteoclast function, leading to more effective and tailored therapies for bone-related disorders. |
| Research on Bone Physiology and Pathology |
Significance: Osteoclast markers are essential for advancing our understanding of bone physiology and pathology. Studying these markers helps elucidate the complex interactions between osteoclasts, osteoblasts, and the bone microenvironment. Impact: Research on osteoclast markers contributes to the discovery of novel signaling pathways, regulatory mechanisms, and therapeutic targets involved in bone metabolism, providing insights into the development of innovative approaches for managing bone diseases. |
| Personalized Medicine |
Significance: Identifying and studying osteoclast markers can contribute to the development of personalized treatment strategies for individuals with bone disorders. By analyzing specific markers, clinicians can tailor therapies to target the underlying mechanisms of bone pathology in each patient. Impact: Personalized medicine approaches based on osteoclast markers can improve treatment outcomes, reduce side effects, and optimize patient care by considering individual variations in osteoclast activity and responsiveness to therapy. |
In conclusion, the identification and study of osteoclast markers play a crucial role in advancing research, diagnosis, treatment, and personalized management of bone-related disorders. By understanding the expression and function of these markers, researchers and clinicians can unravel the complexities of bone metabolism, develop targeted therapies, and improve patient outcomes in various clinical settings.
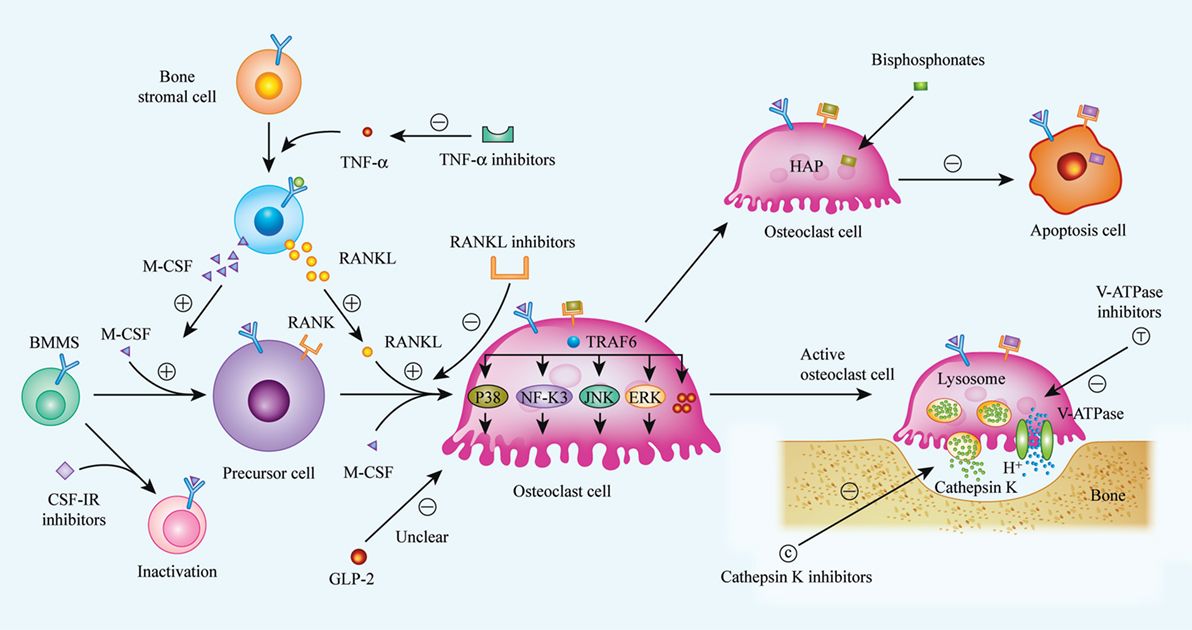 Fig.1 Biological procedures of osteoclast differentiation, bone resorption, and mechanisms of current or future therapeutic drugs. (Bi H, et al., 2017)
Fig.1 Biological procedures of osteoclast differentiation, bone resorption, and mechanisms of current or future therapeutic drugs. (Bi H, et al., 2017)Applications of Osteoclast Markers in Each Disease
The expression levels of osteoclast markers can serve as valuable diagnostic and prognostic indicators in conditions such as osteoporosis, rheumatoid arthritis, and bone metastasis. Here's how these markers can be utilized in each of these conditions:
| Disease | Application | Descriptions |
|---|---|---|
| Osteoporosis | Diagnostic Indicators |
TRAP, Cathepsin K, ALP: Increased expression levels of these markers can indicate heightened osteoclast activity, which is a hallmark of bone resorption in osteoporosis. RANKL: Elevated RANKL levels relative to osteoprotegerin (OPG) can signify an imbalance favoring bone resorption over formation. |
| Prognostic Indicators |
SOST: Elevated levels of SOST, an inhibitor of the Wnt signaling pathway, can indicate reduced bone formation and potentially predict worsening bone density. ICTP, CTX: Elevated levels of C-terminal telopeptide of type I collagen (ICTP) and C-terminal telopeptide (CTX) can predict bone resorption rates and fracture risk. |
|
| Rheumatoid Arthritis | Diagnostic Indicators |
CD44, RANK, RANKL: Increased expression of these markers can indicate osteoclast activation in the bone erosion process seen in rheumatoid arthritis. MMP-9: Matrix metalloproteinase-9, associated with osteoclast activity, can be elevated in the presence of bone resorption. |
| Prognostic Indicators |
TRAP, CTR: High levels of tartrate-resistant acid phosphatase (TRAP) and calcitonin receptor (CTR) can predict joint damage and disease progression. Osteopontin, Osteocalcin: Altered levels of these markers can indicate changes in bone turnover and predict bone density changes over time. |
|
| Bone Metastasis | Diagnostic Indicators |
PTHrP, RANKL: Tumor cells can produce factors like parathyroid hormone-related protein (PTHrP) and RANKL, leading to increased osteoclast activity and bone destruction in metastatic sites. Dkk1: Elevated Dickkopf-1 levels can interfere with Wnt signaling in osteoblasts, contributing to bone resorption in metastatic lesions. |
| Prognostic Indicators |
ALP, Bone Sialoprotein, Osteopontin: Altered levels of these markers can indicate tumor-induced changes in the bone microenvironment and predict bone metastasis progression. |
Overall Impact
- Diagnostic Utility: Monitoring osteoclast marker levels can aid in the early detection and monitoring of bone-related pathologies.
- Prognostic Value: Changes in marker expression levels can help predict disease progression, treatment response, and fracture risk.
- Therapeutic Targets: Targeting these markers pharmacologically can help modulate osteoclast activity and bone remodeling in these conditions.
By leveraging the expression profiles of osteoclast markers, clinicians can gain insights into the underlying bone dynamics in these conditions, enabling more personalized and effective management strategies for patients with osteoporosis, rheumatoid arthritis, and bone metastasis.
Case Study
Case 1: Hulley PA, Knowles HJ. A New Method to sort differentiating osteoclasts into defined homogeneous subgroups. Cells. 2022; 11(24):3973.
Osteoclasts play a crucial role in regulating skeletal development and are key drivers of pathological osteolysis, making them attractive targets for therapeutic interventions. However, research on osteoclasts faces challenges due to the heterogeneity of cell populations generated in vitro. Traditionally, a mixture of undifferentiated monocytes, binuclear pre-osteoclasts, and multinucleated osteoclasts has been treated as a single population.
A recent study introduces a method for differentiating primary human CD14+ monocyte-derived osteoclasts in 3D collagen gels. These osteoclasts, although small (with most having ≤5 nuclei), were viable and functionally active. Upon release from the gel using collagenase, they rapidly fused when reseeded on solid substrates and exhibited dentine resorption capabilities for several weeks.
These 3D-generated osteoclasts expressed specific cell surface markers associated with osteoclast differentiation, such as CD9, RANK, OSCAR, CD63, and CD51/61. Their small size facilitated live cell sorting, enabling the isolation of highly enriched viable subpopulations of human osteoclasts with full functional resorption capacity. By effectively removing undifferentiated cells, low-yield osteoclast preparations were enriched, demonstrating a significant increase in differentiated cells.
This innovative approach allows for the study of selected populations of differentiating osteoclasts in vitro, paving the way for comprehensive transcriptomic and proteomic analyses. This advancement enhances our understanding of human osteoclast molecular mechanisms relevant to various processes, including development, aging, and disease.
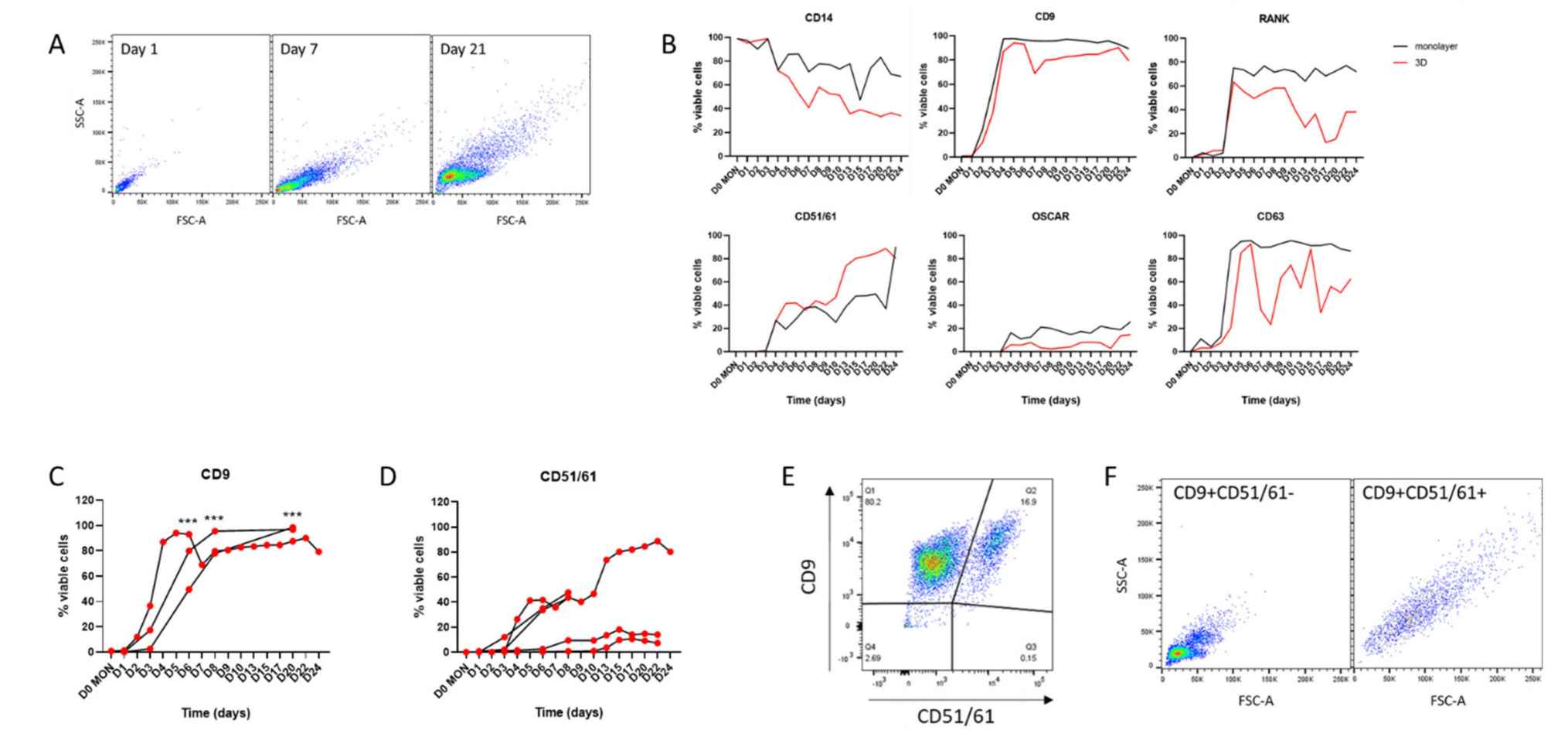 Fig.1 Cell surface markers in monolayer and 3D osteoclasts.
Fig.1 Cell surface markers in monolayer and 3D osteoclasts.Case 2: Li S, Hao L, Wang L, et al. Targeting Atp6v1c1 prevents inflammation and bone erosion caused by periodontitis and reveals its critical function in osteoimmunology. PLoS One. 2015;10(8):e0134903.
In the study discussed, researchers investigated the efficacy of a novel adeno-associated virus (AAV)-mediated Atp6v1c1 knockdown gene therapy for addressing bone erosion and inflammation associated with periodontitis in a mouse model. Atp6v1c1, a subunit of the V-ATPase complex crucial for osteoclast-mediated bone resorption, was targeted due to its essential role in this process. The hypothesis was that by targeting Atp6v1c1, the bone erosion and inflammation characteristic of periodontitis could be effectively mitigated.
Through AAV RNA interference (RNAi) knockdown of the Atp6v1c1 gene, the researchers aimed to simultaneously prevent bone erosion and gingival inflammation. The results showed that site-specific injection of AAV-shRNA-Atp6v1c1 into the periodontal lesions significantly protected against bone erosion (over 85%) and gingival inflammation induced by P. gingivalis W50 infection. This knockdown approach notably reduced osteoclast numbers, thwarted the infiltration of dendritic cells and macrophages in the inflammatory lesions triggered by bacteria in periodontitis, and prevented the expression of osteoclast-related genes and pro-inflammatory cytokine genes.
The data from this study strongly suggests that AAV-shRNA-Atp6v1c1 treatment effectively attenuates both bone erosion and inflammation associated with periodontitis. This highlights the dual functionality of AAV-shRNA-Atp6v1c1 as an inhibitor of bone erosion mediated by osteoclasts and as a suppressor of inflammation by down-regulating pro-inflammatory cytokine expression. Overall, the findings support the potential of Atp6v1c1 RNAi knockdown gene therapy delivered via AAV-shRNA-Atp6v1c1 as a promising and innovative therapeutic approach for addressing bone erosion and inflammatory conditions like periodontitis and rheumatoid arthritis.
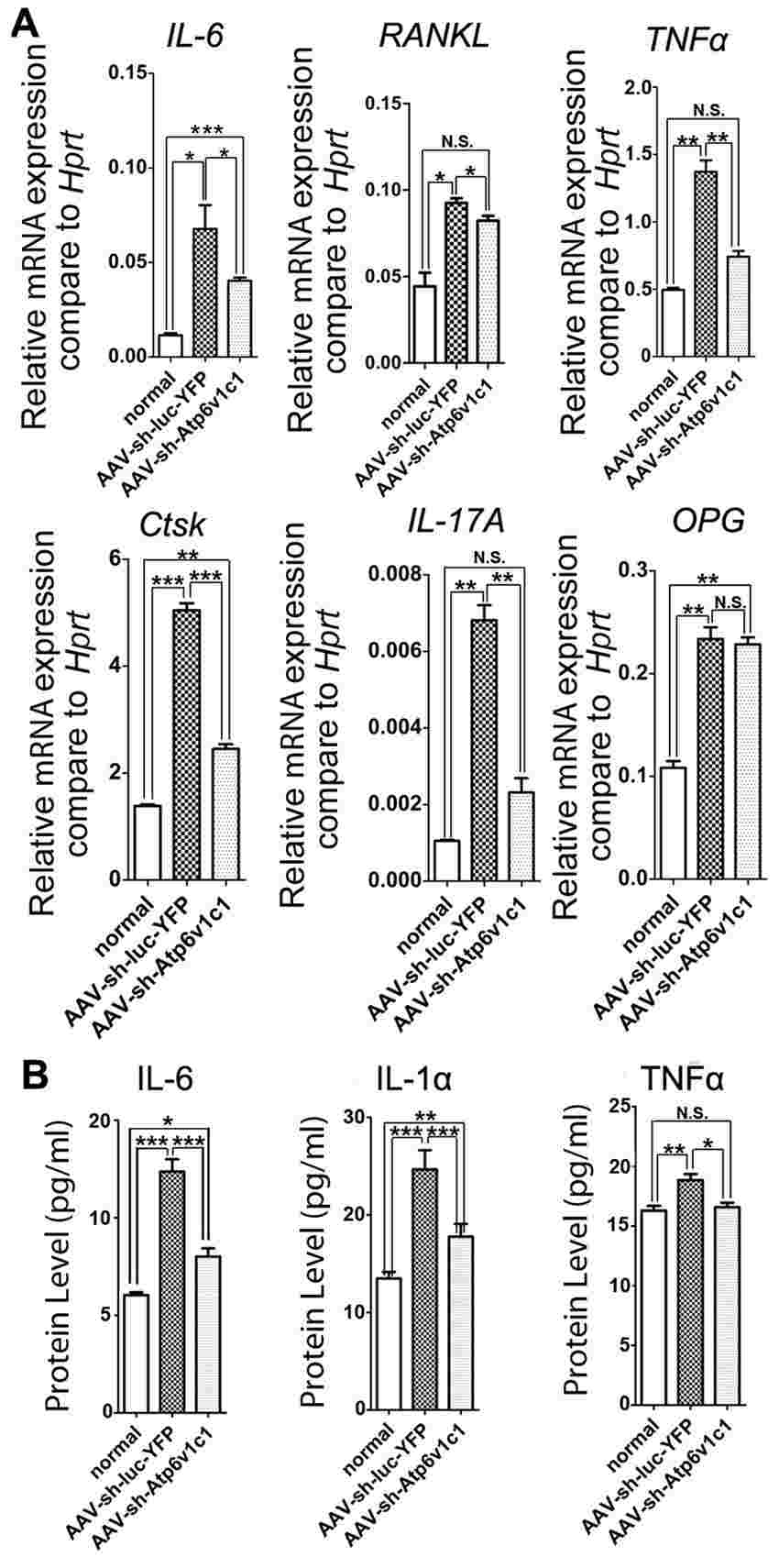 Fig.2 The expression of osteoclast marker genes and cytokines in the periodontal tissues were reduced by AAV-sh-Atp6v1c1.
Fig.2 The expression of osteoclast marker genes and cytokines in the periodontal tissues were reduced by AAV-sh-Atp6v1c1.Case 3: Vas, V., Kovács, T., Körmendi, S. et al. Significance of the Tks4 scaffold protein in bone tissue homeostasis. Sci Rep 9, 5781 (2019).
The main driver of osteoporosis is an imbalance between bone resorption and formation, with genetic alterations in key osteogenic factors and dysfunction of bone marrow mesenchymal stem/stromal cells (BM-MSCs) contributing to its pathogenesis. Tks4, a scaffold protein encoded by the Sh3pxd2b gene, is crucial for podosome organization. Homozygous mutational inactivation of Sh3pxd2b leads to Frank-ter Haar syndrome (FTHS), impacting bone tissue along with eye, ear, and heart functions. The study aimed to investigate the role of Tks4 in adult bone homeostasis by analyzing facial and femoral bone phenotypes in Sh3pxd2b knock-out (KO) mice using micro-CT methods, alongside examining the bone microstructure of an FTHS patient.
Macro-examination of skulls from Tks4-deficient mice revealed craniofacial malformations similar to FTHS symptoms. Femurs of Sh3pxd2b-KO mice exhibited trabecular system alterations indicative of osteoporosis, mirroring increased trabecular separation/porosity in the FTHS patient. Reduced expression levels of bone formation markers Runx2 and osteocalcin were observed in the bone and bone marrow of Sh3pxd2b-KO femurs, respectively. Recent findings indicated that Sh3pxd2b-KO BM-MSCs showed diminished osteoblast lineage differentiation capacity, suggesting the importance of the Tks4 scaffold protein in osteoblast formation and bone cell homeostasis.
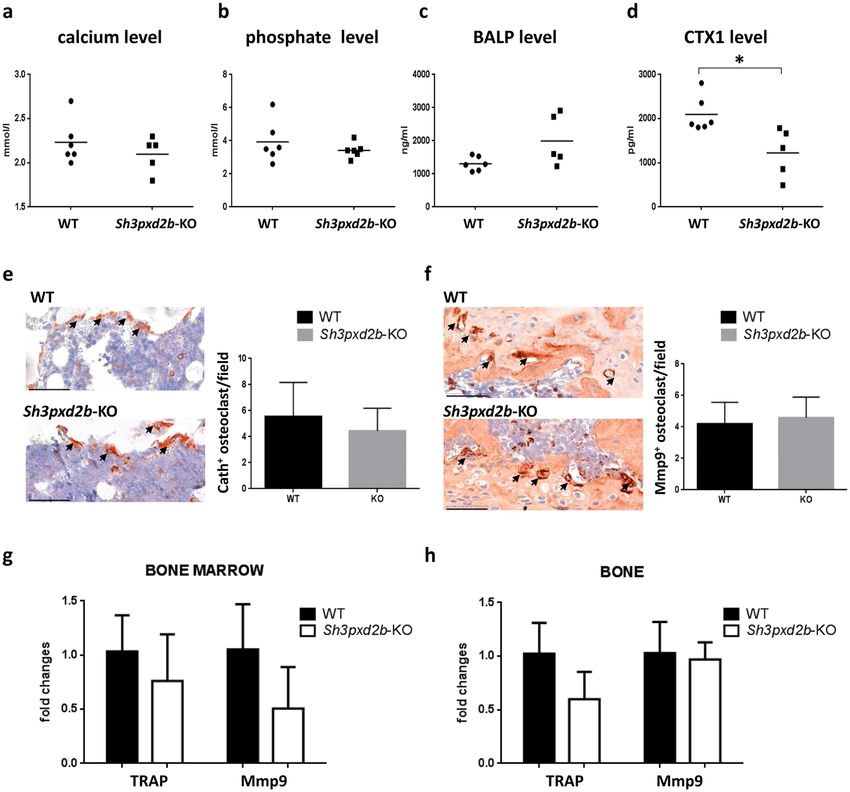 Fig.3 Examination of osteoclast markers.
Fig.3 Examination of osteoclast markers.Related References
- Hulley PA, Knowles HJ. A New Method to sort differentiating osteoclasts into defined homogeneous subgroups. Cells. 2022; 11(24):3973.
- Muruganandan S, Ionescu AM, Sinal CJ. At the crossroads of the adipocyte and osteoclast differentiation programs: future therapeutic perspectives. International Journal of Molecular Sciences. 2020; 21(7):2277.
- Bi H, Chen X, Gao S, et al. Key Triggers of osteoclast-related diseases and available strategies for targeted therapies: a review. Front Med (Lausanne). 2017;4:234.

