Active Recombinant Human BMP2 protein
| Cat.No. : | BMP2-01H |
| Product Overview : | Recombinant Human BMP2 protein was expressed in Escherichia coli. |
| Availability | January 25, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Citation
- Case Study
- Application
- Download
| Gene Name | BMP2 |
| Official Symbol | BMP2 |
| Synonyms | BMP2; bone morphogenetic protein 2; BMP2A; BMP-2A; bone morphogenetic protein 2A; BDA2; |
| Gene ID | 650 |
| mRNA Refseq | NM_001200 |
| Protein Refseq | NP_001191 |
| MIM | 112261 |
| UniProt ID | P12643 |
| ◆ Recombinant Proteins | ||
| BMP2-260H | Recombinant Human BMP2 Protein, GST-tagged | +Inquiry |
| BMP2-26315TH | Recombinant Human BMP2 | +Inquiry |
| BMP2-210H | Recombinant Human BMP2 protein, His/S-tagged | +Inquiry |
| BMP2-558D | Recombinant Dog BMP2 protein, His-tagged | +Inquiry |
| BMP2-557C | Recombinant Cattle BMP2 protein, His & T7-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| BMP2-3075HCL | Recombinant Human BMP2 cell lysate | +Inquiry |
JAGGED1 Stimulates Cranial Neural Crest Cell Osteoblast Commitment Pathways and Bone Regeneration Independent of Canonical NOTCH Signaling
Journal: Bone PubMed ID: 32980561 Data: 2022/4/24
Authors: Archana Kamalakar, Jay M. McKinney, Steven L. Goudy
Article Snippet:O9–1 cells were cultured in 24-well culture plates.O9–1 cells were cultured in 24-well culture plates.. When cells reached 100% confluency, they were treated (n = 3) for 12 hours with Fc-dynabeads (5.7 μM), unbound BMP2 (100 nM) (Creative BioMart, BMP2–01H) and JAG1-dynabeads (5.7 μM) with or without DAPT (15 μM) (Millipore Sigma, D5942).. After 12 hours, RNA was isolated using the Qiagen RNeasy kit (Qiagen, 74106) according to manufacturer’s protocols.After 12 hours, RNA was isolated using the Qiagen RNeasy kit (Qiagen, 74106) according to manufacturer’s protocols.
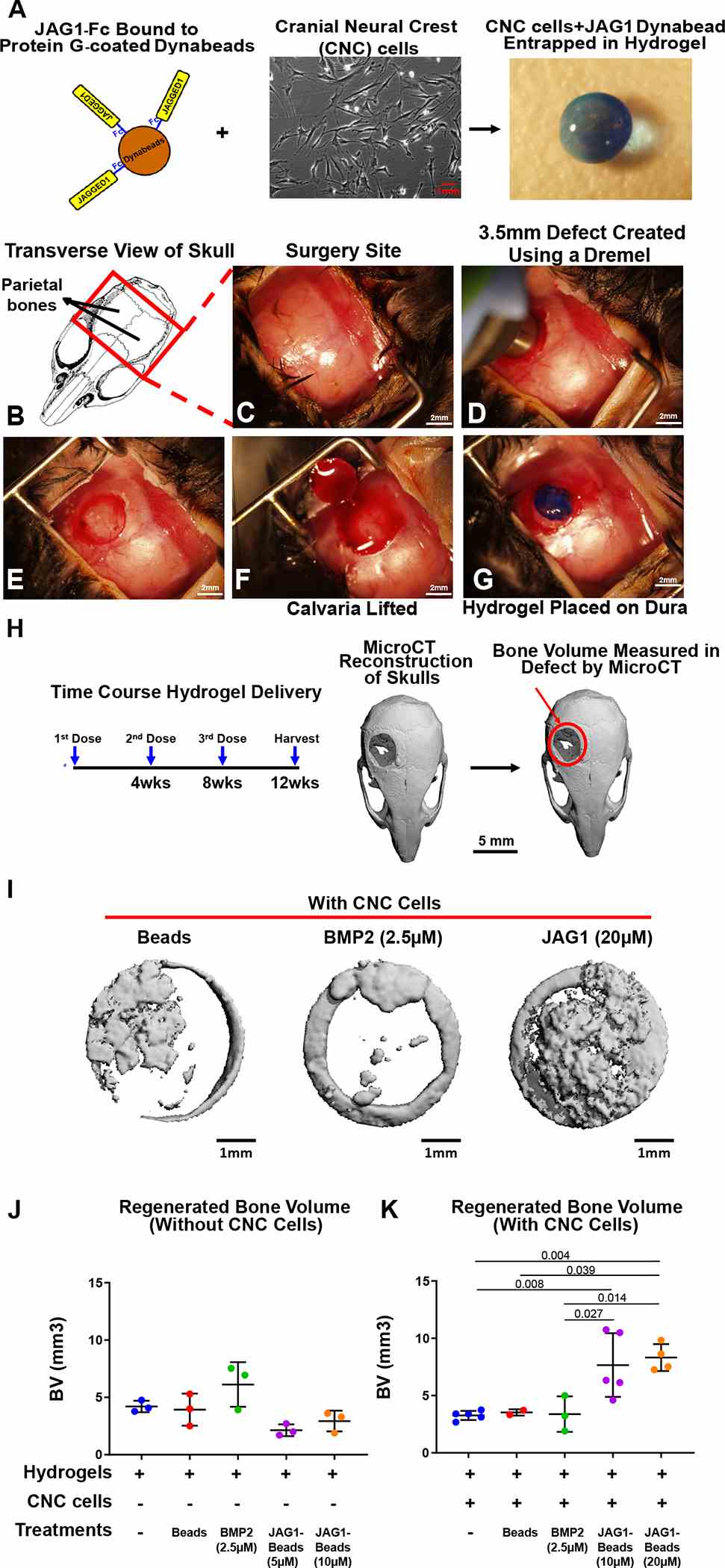
As a proof of concept experiment, we (A) incorporated JAG1-dynabeads complex (5 μM, 10 μM or 20 μM), dynabeads alone and
A non-canonical JAGGED1 signal to JAK2 mediates osteoblast commitment in cranial neural crest cells
Journal: Cellular signalling PubMed ID: 30529759 Data: 2020/2/1
Authors: Archana Kamalakar, Melissa S. Oh, Steven L. Goudy
Article Snippet:These cells were grown and maintained in DMEM (Coming 10-013-CV) + 10% FBS + 1% antibiotics.These cells were grown and maintained in DMEM (Coming 10-013-CV) + 10% FBS + 1% antibiotics.. Osteogenic cultures: The capacity of O9-1 cells to mineralize the surrounding matrix was tested by providing osteogenic media (OM): β-MEM containing 10% FBS, 1% penicillin/streptomycin, 100μg/ml ascorbic acid (Fisher, A62-500), 5 mM β-glycerophosphate (Sigma, G9422-50G) and 100ng/ml Bone morphogenetic protein-2 BMP2 (Creative BioMart, BMP2-01H).. To assure that osteogenic media was essential for matrix mineralization, control wells were incubated in growth media (GM): α-MEM containing 10% FBS and 1% penicillin/streptomycin.To assure that osteogenic media was essential for matrix mineralization, control wells were incubated in growth media (GM): α-MEM containing 10% FBS and 1% penicillin/streptomycin.
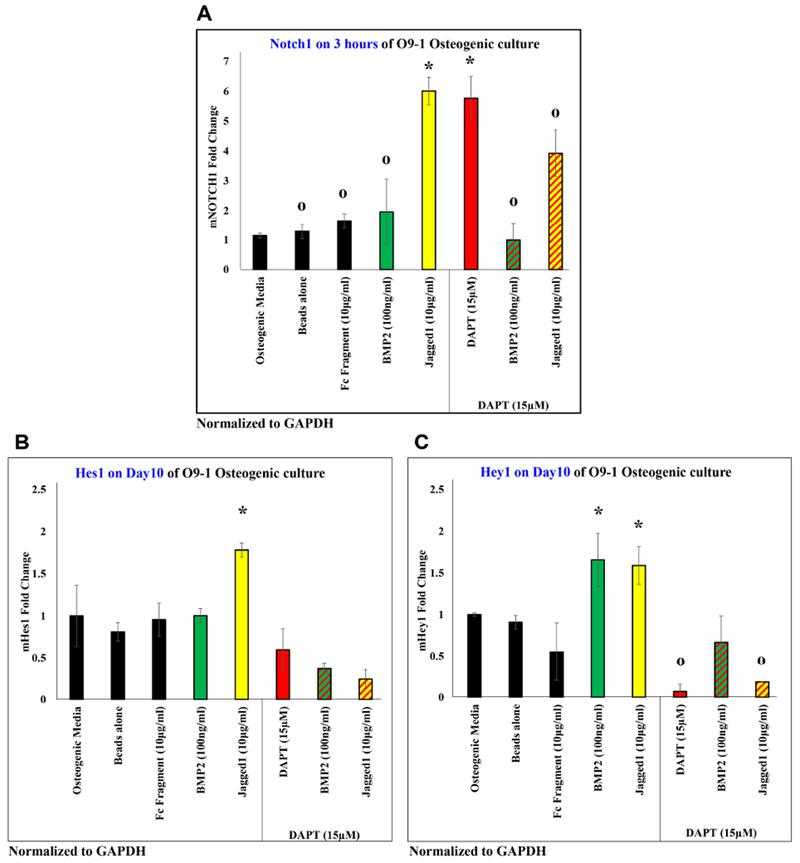
JAGGED1 induces the NOTCH pathway, by inducing expression of Notch1 receptor in (A), and expression of classic NOTCH targets (B) Hes1 and (C) Hey1. The expression of all genes induced by JAG1 was observed to be inhibited by a canonical NOTCH pathway inhibitor, DAPT (red bar, red patterned bars). Dynabeads bound recombinant JAG1-Fc fragment (yellow) or
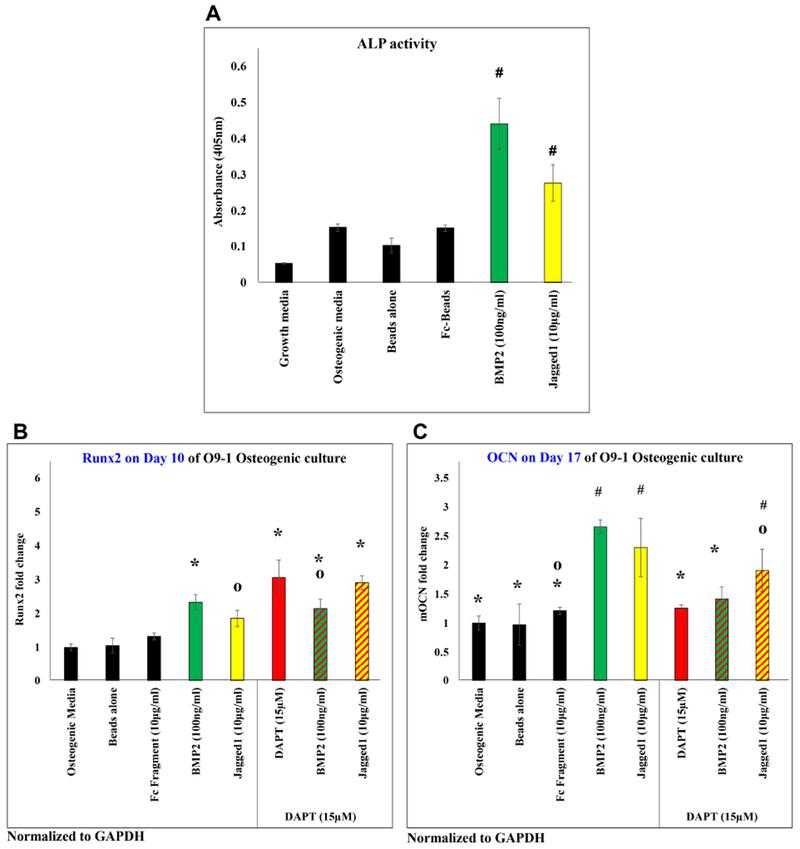
JAGGED1 induces osteoblastogenesis, as demonstrated by measuring the (A) alkaline phosphatase activity of O9-1 cells when treated with dynabeads bound recombinant JAG1-Fc fragment (yellow) or
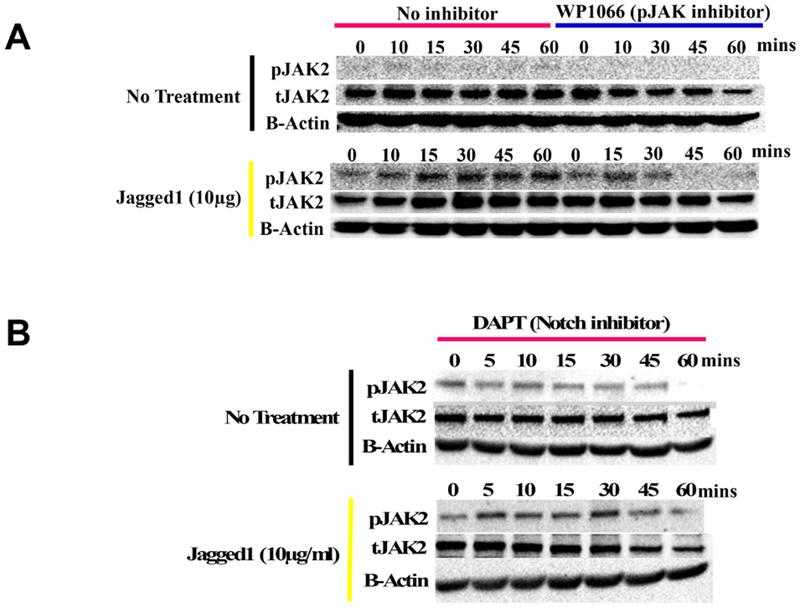
(A) Lysates obtained from O9-1 cells treated with dynabeads alone (entire blots in Supplemental Figure S4A) or bound to Fc-fragment,
Osseointegration of dental implants in ectopic engineered bone in three different scaffold materials.
Journal: International journal of oral and maxillofacial surgery PubMed ID: 31053519 Data: 2020/1/2
Authors: H Naujokat, Y A?il, J Wiltfang
Article Snippet:The non-degradable scaffold consisted of TiAl6V4 titanium alloy and was manufactured by selective laser melting with a strut thickness of 0.5 mm and a porosity of 70% (Materialise NV, Leuven, Belgium); two cylindrical perforations with a diameter of 4 mm determined the implant position and these were filled with a collagen sponge (Parasorb Cone; Resorba Medical GmbH, Nu?rnberg, Germany).a strut thickness of 0.5 mm and a porosity of 70% (Materialise NV, Leuven, Belgium); two cylindrical perforations with a diameter of 4 mm determined the implant position and these were filled with a collagen sponge (Parasorb Cone; Resorba Medical GmbH, Nu?rnberg, Germany). ... The scaffolds were loaded with 5 ml of autogenous bone marrow aspirate from the iliac crest and 250 mg of recombinant human bone morphogenetic protein type 2 (rhBMP-2, Cat. No. MBP2-01H; Creative BioMart, Shirley, NY, USA) and wrapped in a degradable collagen membrane (BioGide; Geistlich Pharma AG, Wolhusen, Switzerland).. Anaesthesia was induced by intramuscular injection of 10 mg/kg ketamine and 1.5 mg/kg midazolam and maintained by intravenous titration.Anaesthesia was induced by intramuscular injection of 10 mg/kg ketamine and 1.5 mg/kg midazolam and maintained by intravenous titration.
Bone tissue engineering in the greater omentum is enhanced by a periosteal transplant in a miniature pig model.
Journal: Regenerative medicine PubMed ID: 30764722 Data: 2019/5/20
Authors: Hendrik Naujokat, Maximilian Lipp, J?rg Wiltfang
Article Snippet:The specific surface area was up to 60 m2/g, and the pore size was 150 ± 50 μm with complete interconnectivity [24].The specific surface area was up to 60 m2/g, and the pore size was 150 ± 50 μm with complete interconnectivity [24].. Before implantation, blocks were soaked with 5 ml of autogenous bone marrow aspirate and 250 μg of recombinant human BMP type-2 (rhBMP-2, Cat. No. MBP2-01H, Creative BioMart, NY, USA) under sterile conditions.. Bone marrow aspirate was acquired by puncture of the posterior iliac crest and collected in a syringe with 1500 IE heparin.Bone marrow aspirate was acquired by puncture of the posterior iliac crest and collected in a syringe with 1500 IE heparin.
Biofunctionalizing devitalized bone allografts through polymer-mediated short and long term growth factor delivery.
Journal: Journal of biomedical materials research. Part A PubMed ID: 25689463 Data: 2016/5/2
Authors: Farzana Sharmin, Douglas Adams, Yusuf Khan
Article Snippet:Polymer coating volume was quantified by analyzing the microCT images.Polymer coating volume was quantified by analyzing the microCT images.. Growth factor loading and release Recombinant human BMP-2 (Creative BioMart, NY) was loaded onto polymer-coated allografts through surface adsorption or encapsulation while VEGF (sigma Aldrich, MO) was loaded onto separate polymer-coated allografts through surface adsorption alone.. BMP-2 was encapsulated within the polymer coating by adding a concentrated solution of the growth factor (500 lg/mL)33 to the dissolved polymer prior to allograft coating and then coating as described above.BMP-2 was encapsulated within the polymer coating by adding a concentrated solution of the growth factor (500 lg/mL)33 to the dissolved polymer prior to allograft coating and then coating as described above.
Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1 Osx ?/? mice
Journal: Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research PubMed ID: 25043591 Data: 2016/1/1
Authors: Jean de la Croix Ndong, David M. Stevens, Florent Elefteriou
Article Snippet:Once stirring was completely impeded, the crude viscous polymer product was dissolved in a minimal amount of methanol and precipitated into vigorously stirring acetone, which was then decanted to afford the pure glycidol homopolymer product as a translucent, viscous material.Once stirring was completely impeded, the crude viscous polymer product was dissolved in a minimal amount of methanol and precipitated into vigorously stirring acetone, which was then decanted to afford the pure glycidol homopolymer product as a translucent, viscous material.. Recombinant human bone morphogenic protein-2 (rBMP2, Creative BioMart) was suspended in 20 mM acetic acid (10 mg/mL), added to polyglycidol, and diluted with PBS for a rBMP2 concentration of 0.50 μg/μL ( 6 ) and polyglycidol concentration of 0.46 g/mL.. For Trametinibin jections and combo injections, encapsulated Trametinib was first sonicated in PBS and transferred to the polyglycidol mix to yield a Trametinib concentration of 0.188 μg/μL ( 17 , 18 ).For Trametinibin jections and combo injections, encapsulated Trametinib was first sonicated in PBS and transferred to the polyglycidol mix to yield a Trametinib concentration of 0.188 μg/μL ( 17 , 18 ).
A non-canonical JAGGED1 signal to JAK2 mediates osteoblast commitment in cranial neural crest cells
Journal: Cellular signalling PubMed ID: 30529759 Data: 2020/2/1
Authors: Archana Kamalakar, Melissa S. Oh, Steven L. Goudy
Article Snippet:These cells were grown and maintained in DMEM (Coming 10-013-CV) + 10% FBS + 1% antibiotics.These cells were grown and maintained in DMEM (Coming 10-013-CV) + 10% FBS + 1% antibiotics.. Osteogenic cultures: The capacity of O9-1 cells to mineralize the surrounding matrix was tested by providing osteogenic media (OM): β-MEM containing 10% FBS, 1% penicillin/streptomycin, 100μg/ml ascorbic acid (Fisher, A62-500), 5 mM β-glycerophosphate (Sigma, G9422-50G) and 100ng/ml Bone morphogenetic protein-2 BMP2 (Creative BioMart, BMP2-01H).. To assure that osteogenic media was essential for matrix mineralization, control wells were incubated in growth media (GM): α-MEM containing 10% FBS and 1% penicillin/streptomycin.To assure that osteogenic media was essential for matrix mineralization, control wells were incubated in growth media (GM): α-MEM containing 10% FBS and 1% penicillin/streptomycin.

JAGGED1 induces the NOTCH pathway, by inducing expression of Notch1 receptor in (A), and expression of classic NOTCH targets (B) Hes1 and (C) Hey1. The expression of all genes induced by JAG1 was observed to be inhibited by a canonical NOTCH pathway inhibitor, DAPT (red bar, red patterned bars). Dynabeads bound recombinant JAG1-Fc fragment (yellow) or

(A) Lysates obtained from O9-1 cells treated with dynabeads alone (entire blots in Supplemental Figure S4A) or bound to Fc-fragment,
Case1: Hassan AH, et al. J Liposome Res. 2016.
The aim of the present study was to develop and examine a new non-invasive injectable graft for the repair of alveolar bone clefts using recombinant human bone morphogenetic protein-2 (rhBMP-2) encapsulated within injectable liposomal in situ gel (LIG).
Different liposomal formulations loaded with rhBMP-2 were prepared, and the effects of the preparation methods and lipid content on the efficiency of rhBMP-2 encapsulation within the liposomes were studied.
The results indicated that the prepared rhBMP-2-LIG prolonged the release and residence time of BMP-2 within rabbits for more than 7 days.
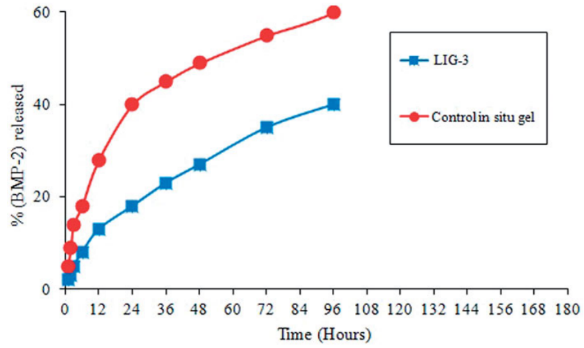
Fig1. In vitro (BMP-2) release profile from LIG formulations (LIG-3) and control in situ gel.
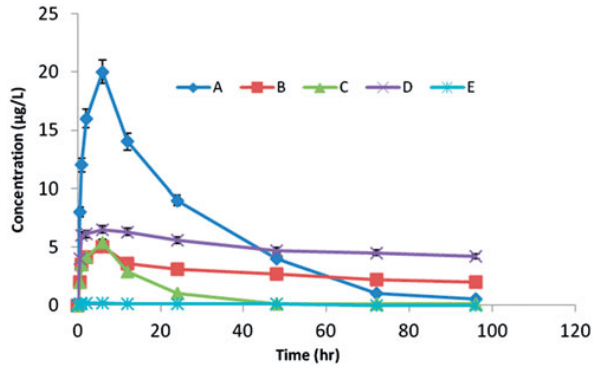
Fig2. Mean plasma levels of BMP-2 after the local injection of 5 mg/kg of BMP-2 isotonic saline into defects in rabbits (A), BMP-2 in unilamellar liposomal suspension (B), BMP-2 dispersed in the in situ gel (C), rhBMP-2-LIG-3 formula (D) and negative control (E).
Case2: Janki Jayesh Patel, et al. Tissue Engineering Part C: Methods, 2015
In this study, EPO and BMP2 are adsorbed separately on two 3D-printed PCL scaffold modules that are assembled for codelivery on a single scaffold structure. This assembled modular PCL scaffold with dual BMP2 and EPO delivery was shown to increase bone growth in an ectopic location when compared with BMP2 delivery along a replicate scaffold structure.
Dual delivery produced more dense cellular marrow, while BMP2 had more fatty marrow. Dual EPO and BMP2 delivery is a potential method to regenerate bone faster for prefabricated flaps.
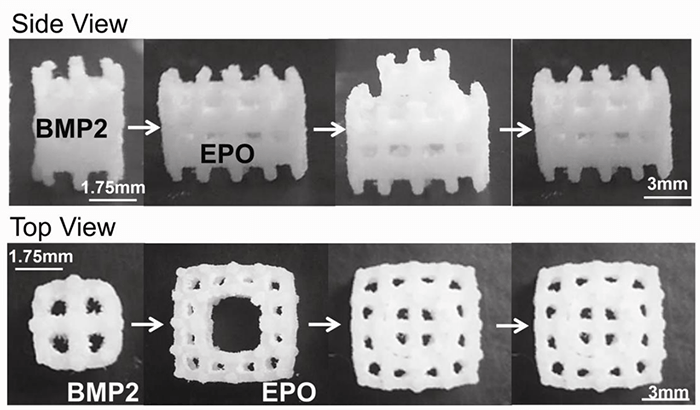
Fig1. Modular Scaffold Assembly. BMP2 was adsorbed onto the inner scaffold module and EPO was adsorbed onto the outer scaffold module. The two scaffolds were then assembled.
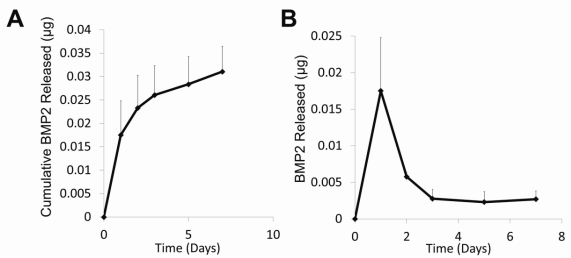
Fig2. 2. Protein Release Profiles. A) BMP2 cumulative release profile. After 7 days, 0.0311±0.0053µg BMP2 was released into PBS at 37oC. B) BMP2 release. A small burst release occurred in the first two days followed by sustained release.
Case3: Alissa, M., et al. Frontiers in Pharmacology, 2023.
The primary purpose of this study is to use phospholipids-based phase separation in-situ gel (PPSG) in combination with bone morphogenetic protein-2 nanoemulsion (BMP-2-NE) to aid repairing alveolar bone defects.
Injectable PPSG with the best NE formulation was tested for viscosity characteristics, gel strength, water absorption, and in-vitro BMP-2 release.
PPSG that has been loaded with BMP-2 NE may therefore be a promising, fruitful, and less painful paradigm for the noninvasive therapy of CLP with significant effect and extended release.
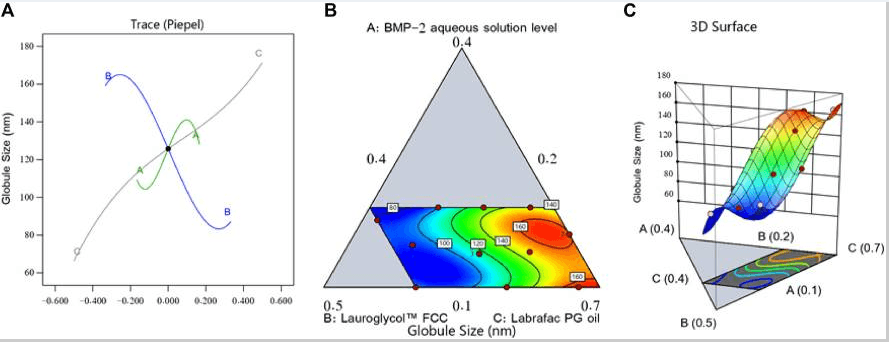
Fig1. Perturbation outline (A), contour diagram (B), and 3D surface graph (C) showing the effects of different factors on the droplet size (Y1) of different BMP-2-NE formulations.
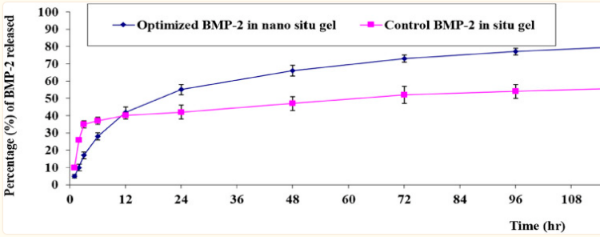
Fig2. In-vitro release behavior of BMP-2 aqueous solution from the optimal formulation of the loaded gel compared with the control BMP-2 solution of the loaded gel (mean ± SD, n = 3).
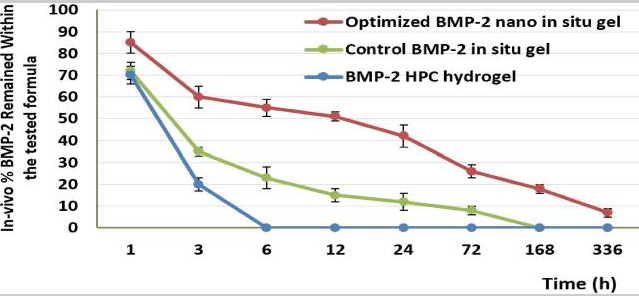
Fig3. The in-vivo release percentage of BMP-2 in the maxilla for different treatment groups after 14 days.
Recombinant Human Bone Morphogenetic Protein-2 (rhBMP2), a versatile bioengineered protein, is a finely recreated version of the naturally occurring protein in human bodies that significantly contributes to the bone and cartilage formation. In this article, we delve into the various applications of rhBMP2, exhibiting its intricate functionalities in clinical and therapeutic settings.
One of the most prominent areas of application of rhBMP2 lies within orthopedic surgery and dental procedures. The protein is noted for its remarkable capability to stimulate bone growth, efficiently filling up gaps in bones resulting from injury or disease. This has resulted in its extensive usage for spinal fusion surgeries, where it has shown remarkable results in supporting the bone growth needed for the effective fusion of vertebrae. The rhBMP2 protein has also gained popularity in oral and maxillofacial surgery. Its ability to enhance bone growth has been leveraged to support dental implants and treat periodontal diseases.
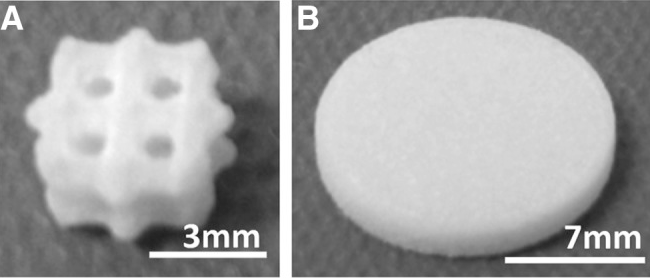
Fig1. (A) PCL scaffold (B) PCL disc. PCL, poly-?-caprolactone.
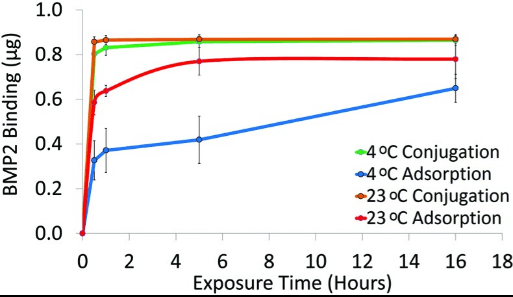
Fig2. BMP2 binding to PCL discs via adsorption or conjugation. PCL discs were exposed to 1.4?μg/mL BMP2 solution for0.5, 1, 5, or 16?h at 23°C or 4°C. BMP2 was quantified with an ELISA (n=3). BMP2, bone morphogenetic protein-2; ELISA,enzyme-linked immunoabsorbant assay.
Another important application of rhBMP2 is in the treatment of complex fractures. Often, certain non-union fractures fail to heal with standard treatments and result in long term pain and disability. In such cases, rhBMP2 is utilized to stimulate bone growth and accelerate the healing process. The protein is adeptly engineered into a sponge that is placed at the fracture site during surgery. This then releases the protein over time, thus stimulating bone formation and healing.
Recombinant BMP2 has also shown promising results in the field of tissue engineering for the generation of new bone tissues. Research in this domain is primarily focusing on combining rhBMP2 with various biomaterials to act as bone graft substitutes, which can be used in bone regeneration therapies. These grafts that incorporate rhBMP2 have shown superior efficacy in promoting bone formation as compared to those without rhBMP2.
Apart from these, rhBMP2 has also shown potential in aiding the healing process for patients battling bone cancer. As bone cancer often needs a high amount of bone grafting after surgery, the application of rhBMP2 promotes faster bone regeneration, thereby accelerating the recovery process for afflicted patients. Furthermore, it reduces the often excessive dependency on autografts, thereby potentially minimizing the risk of donor site morbidity.
While it presents several impressive applications, the usage of rhBMP2 in clinical settings must be regulated with careful consideration. Overdosing or inappropriate administration of the protein can lead to severe side effects, such as ectopic bone formation or even potential immunological responses. Therefore, the dosage and handling of rhBMP2 in any therapeutic application must be accurately monitored.
Recombinant BMP2 has therefore opened up new avenues of treatment in various fields such as orthopedics, dentistry, tissue engineering, and oncology. Its ability to enhance bone formation and healing clearly marks it as a significant medical advancement. With ongoing research and improvements, its wide range of applications could further expand, leading to novel therapeutic approaches in bone-related diseases and surgeries.
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All BMP2 Products
Required fields are marked with *
My Review for All BMP2 Products
Required fields are marked with *



