Human Factor-VIII
| Cat.No. : | F8-3065H |
| Product Overview : | Human Facor VIII produced from Human Plasma is effective in the correction and prevention of severe bleeding episodes attributed to Factor VIII deficiency. The Factor-VIII is purified by proprietary chromatographic techniques. |
| Availability | January 30, 2026 |
| Unit | |
| Price | |
| Qty |
- Specification
- Gene Information
- Related Products
- Case Study
- Application
- Download
| Species : | Human |
| Source : | Human Plasma |
| Tag : | Non |
| Description : | Coagulation factor VIII participates in the intrinsic pathway of blood coagulation; factor VIII is a cofactor for factor IXa which, in the presence of Ca+2 and phospholipids, converts factor X to the activated form Xa. This gene produces two alternatively spliced transcripts. Transcript variant 1 encodes a large glycoprotein, isoform a, which circulates in plasma and associates with von Willebrand factor in a noncovalent complex. This protein undergoes multiple cleavage events. Transcript variant 2 encodes a putative small protein, isoform b, which consists primarily of the phospholipid binding domain of factor VIIIc. This binding domain is essential for coagulant activity. Defects in this gene results in hemophilia A, a common recessive X-linked coagulation disorder. |
| Physical Appearance : | Sterile Filtered White lyophilized (freeze-dried) powder. |
| Formulation : | The lyophilized protein 200IU/ml was lyophilized from a sterile solution containing 1.5% Glycine, 160mM Calcium chloride and 25mM NaCitrate and 25mM NaCl. |
| Solubility : | It is recommended to reconstitute the lyophilized Factor-VIII in sterile 18MΩ-cm H2O at a concentration of 200IU/ml, which can then be further diluted to other aqueous solutions. Make sure that the vial has reached room temperature prior to its reconstitution, otherwise it might precipitate. |
| Stability : | Lyophilized Factor-VIII although stable at room temperature for 1 week, should be stored desiccated between 2-8°C. Upon reconstitution Factor-VIII should be storedat 4°C. |
| Biological Activity : | The potency per mg was tested and found to be 150 Units/mg. |
| Human Virus Test : | The plasma is collected from donors with Hepatitis B vaccinated. Each unit of plasma has been tested for HBsAg, Anti-HIV-1/2 plus O and Anti-HCV by using the imported kits which are approved by Federal Drug Administration (FDA). |
| Usage : | Creative BioMart"s products are furnished for LABORATORY RESEARCH USE ONLY. The product may not be used as drugs, agricultural or pesticidal products, food additives or household chemicals. |
| Publications : |
| Gene Name | F8 coagulation factor VIII, procoagulant component [ Homo sapiens ] |
| Synonyms | F8; coagulation factor VIII, procoagulant component; AHF; F8B; F8C; HEMA; FVIII; DXS1253E; coagulation factor VIII; factor VIII F8B; coagulation factor VIIIc; antihemophilic factor |
| Gene ID | 2157 |
| mRNA Refseq | NM_000132 |
| Protein Refseq | NP_000123 |
| MIM | 306700 |
| UniProt ID | P00451 |
| Chromosome Location | Xq28 |
| Pathway | Complement and coagulation cascades; Hemostasis |
| Function | copper ion binding; metal ion binding; oxidoreductase activity; protein binding |
| ◆ Recombinant Proteins | ||
| F8-2309H | Recombinant Human F8 Protein (Phe2253-Glu2346), N-GST tagged | +Inquiry |
| F8-12626H | Recombinant Human F8, His-tagged | +Inquiry |
| F8-1921M | Recombinant Mouse F8 protein, His-tagged | +Inquiry |
| F8-912C | Recombinant Cattle F8 Protein, His-tagged | +Inquiry |
| F8-1452C | Recombinant Chicken F8 Protein, His-tagged | +Inquiry |
| ◆ Cell & Tissue Lysates | ||
| F8-6482HCL | Recombinant Human F8 293 Cell Lysate | +Inquiry |
Case 1: Walker GE, et al. J Thromb Haemost. 2022
Trousseau sign, which links cancer to thrombosis, indicates that cancer patients have a higher risk of venous thromboembolism (VTE) and that VTE can precede a cancer diagnosis. Factor VIII (FVIII), a coagulation factor with additional functions, is recognized as an independent VTE risk factor in cancer, but the reason for its increased presence is unclear. To explore this, researchers examined bladder cancer tissues, which are prone to VTE, alongside normal bladder tissues for FVIII expression.
In bladder cancer cell lines, the presence and secretion of FVIII were studied. Additionally, the cancer cell line encyclopedia was utilized to assess the mRNA levels of various coagulation factors, including FVIII, in 811 cell lines from different cancer origins. The study investigated the synthesis, secretion, and activity of FVIII in a range of cancer cell lines.
The findings show that while FVIII is present in normal bladder epithelium, its mRNA and protein levels are elevated in bladder cancer, with bladder cancer cells actively producing and secreting bioactive FVIII. Similar patterns were observed in other cancer cell lines, suggesting a potential independent role for FVIII in the pathophysiology of cancer-related thrombosis, separate from other elements of the coagulation process like von Willebrand factor (VWF).
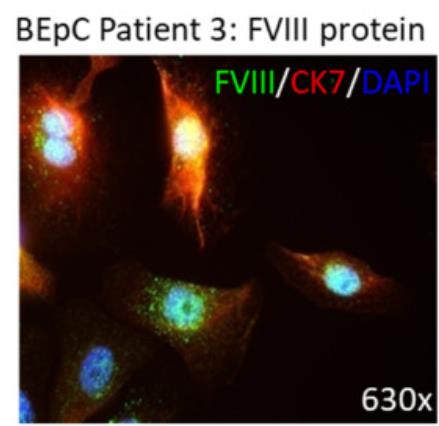
Fig1. Representative immunofluorescence image of FVIII protein costained with CK7 in the isolated bladder epithelial cells.
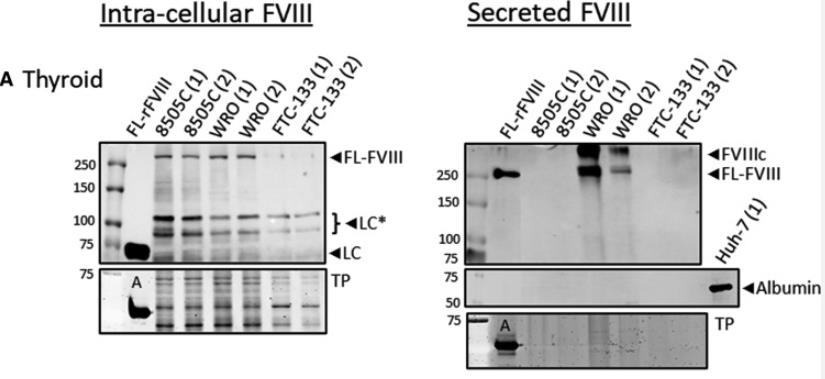
Fig2. Factor VIII is synthesized and secreted by thyroid.
Case 2: Jun Z, et al. Biochem Biophys Res Commun. 2020
Factor VIII (FVIII) is crucial for blood coagulation, aiding in the activation of FX by FIXa. Hemophilia A results from FVIII deficiency. The FVIII gene has 26 exons, encoding a 2351 amino acid protein with domains A1-A2-B-A3-C1-C2. The A and C domains are homologous and essential for FVIII function. The B domain, variable even among species, is highly glycosylated and influences FVIII's lifespan beyond its coagulation role, though its specific functions are unclear.
Researchers created versions of recombinant FVIII (rFVIII) with parts of the B domain removed and expressed them in hepatocytes. After 72 hours, researchers analyzed the biosynthesis, secretion, activity, and stability of these rFVIIIs in plasma, comparing them to full-length FVIII. Interestingly, different segments of the B domain affected these aspects of FVIII in various ways.
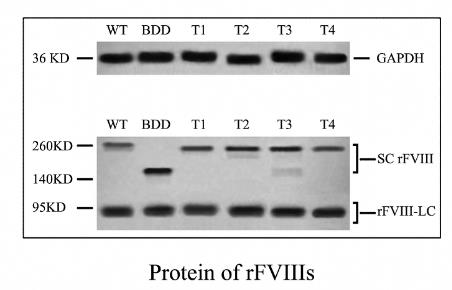
Fig1. The rFVIII protein were qualified with West blot assay.
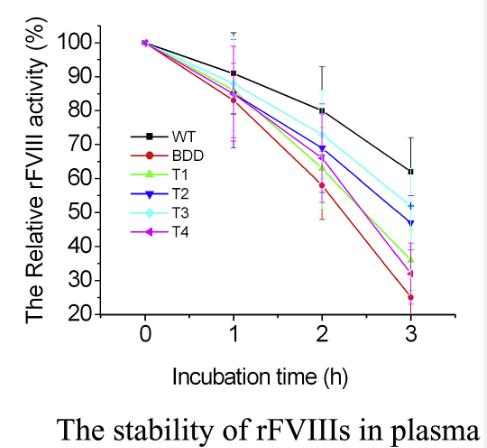
Fig2. In aPTT clotting assay, different incubation time were set to exploit the stability of rFVIIIs in ex vivo plasma.
FVIII is a macromolecular glycoprotein synthesized by the liver and belongs to a clotting factor. Under normal circumstances, FVIII acts as a cofactor to significantly increase the activation rate of clotting factor X, which is achieved through the formation of FVIIIa and FIXa complex, which can effectively activate FX and further promote blood coagulation.
hFVIII is one of the leading alternative therapies to treat hemophilia A, an X-linked recessive bleeding disorder caused by mutations in the FVIII gene. For patients with severe hemophilia A (FVIII activity <1 IU/dL), regular prophylactic therapy with exogenous FVIII is the recommended treatment. This treatment helps to reduce bleeding events and improve quality of life.
Antibodies for the detection of hFVIII can be used to diagnose diseases associated with FVIII, including hemophilia A and other clotting disorders. During drug development, hFVIII and its variants can be used to evaluate the impact of new drugs on FVIII activity, as well as to develop new therapies that may improve treatment outcomes.
Fig1. Bis-Tris SDS PAGE gel of bTSH (product 1) and rhTSH (each 70 μg/lane). (Tirthadipa Pradhan-Sundd, 2021)
Not For Human Consumption!
Inquiry
- Reviews (0)
- Q&As (0)
Ask a Question for All F8 Products
Required fields are marked with *
My Review for All F8 Products
Required fields are marked with *



